Impact of certolizumab pegol on patient-reported outcomes in rheumatoid arthritis and correlation with clinical measures of disease activity
- PMID: 26614481
- PMCID: PMC4662806
- DOI: 10.1186/s13075-015-0849-1
Impact of certolizumab pegol on patient-reported outcomes in rheumatoid arthritis and correlation with clinical measures of disease activity
Abstract
Introduction: The effect of certolizumab pegol (CZP) on patient-reported outcomes (PROs) was investigated in 1063 patients with rheumatoid arthritis (RA) from the REALISTIC trial (double-blind, placebo-controlled to week 12, open-label to week 28; randomized 4:1 [CZP:placebo]). Correlations between PROs and RA signs and symptoms, and the relative efficacy of these measures, were examined.
Methods: Adults with RA and an inadequate response to at least one disease-modifying antirheumatic drug were enrolled. PROs assessed included physical function (using the Health Assessment Questionnaire-Disability Index), pain, fatigue, sleep disturbance, Patient Global Assessment of Disease Activity (PtGA), Routine Assessment of Patient Index Data 3 (RAPID3), and Rheumatoid Arthritis Disease Activity Index (RADAI).
Results: Early significant and clinically meaningful improvements in all PROs were observed to week 12 with CZP vs. placebo and were maintained to the end of the trial (week 28). At week 12, up to one-third more CZP patients showed improvements compared with placebo that were greater than or equal to the minimal clinically important difference (MCID) in fatigue, sleep problems, pain, PtGA, RADAI, and RAPID3. The changes in PROs were correlated with clinical measures of disease activity, including the Disease Activity Score in 28 joints using C-reactive protein as well as tender and swollen joint counts.
Conclusions: Rapid improvements in PROs were seen in patients with RA treated with CZP. The magnitude of improvement exceeded the MCID in multiple domains and demonstrated that CZP improves aspects of health-related quality of life that are meaningful to patients and superior to placebo. PROs provide information complementary to clinical outcomes in assessment of treatment benefits.
Trial registration: ClinicalTrials.gov identifier: NCT00717236 . Registered on 15 July 2008.
Figures
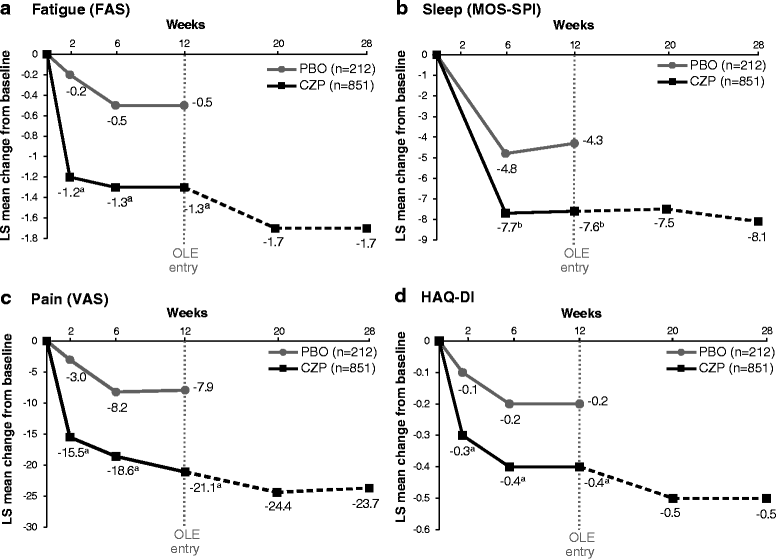

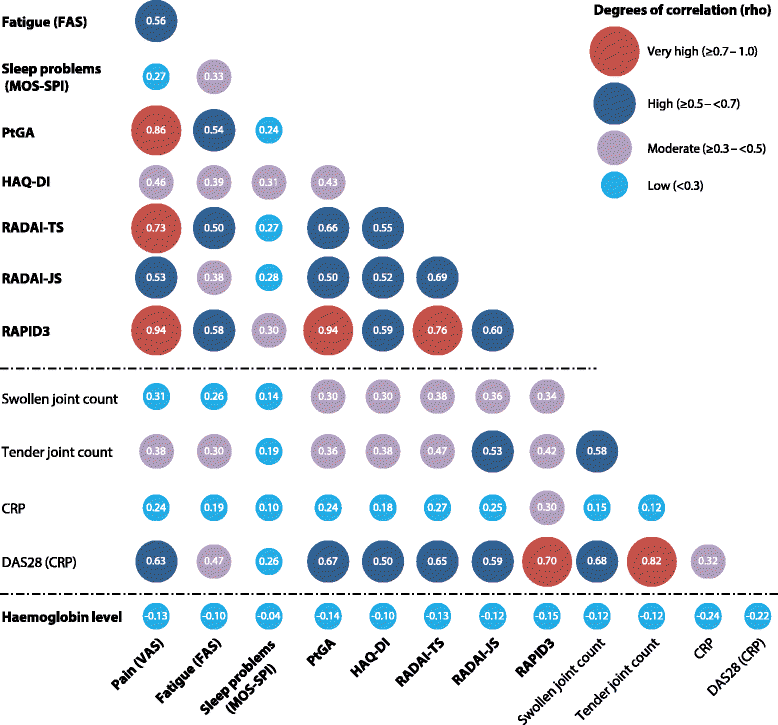
References
-
- Pincus T, Strand V, Koch G, Amara I, Crawford B, Wolfe F, et al. An index of the three core data set patient questionnaire measures distinguishes efficacy of active treatment from that of placebo as effectively as the American College of Rheumatology 20 % response criteria (ACR20) or the Disease Activity Score (DAS) in a rheumatoid arthritis clinical trial. Arthritis Rheum. 2003;48:625–30. doi: 10.1002/art.10824. - DOI - PubMed
-
- Tugwell P, Wells G, Strand V, Maetzel A, Bombardier C, Crawford B, et al. Clinical improvement as reflected in measures of function and health-related quality of life following treatment with leflunomide compared with methotrexate in patients with rheumatoid arthritis: sensitivity and relative efficiency to detect a treatment effect in a twelve-month, placebo-controlled trial. Arthritis Rheum. 2000;43:506–14. A published erratum appears in. Arthritis Rheum. 2000;43:1345. doi: 10.1002/1529-0131(200003)43:3<506::AID-ANR5>3.0.CO;2-U. - DOI - PubMed
-
- U.S. Department of Health and Human Services, FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services, FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services, Center for Devices and Radiological Health Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. doi: 10.1186/1477-7525-4-79. - DOI - PMC - PubMed
-
- Committee for Medicinal Products for Human Use (CHMP). Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. London: European Medicines Agency; 27 Jul 2005. http://www.ispor.org/workpaper/emea-hrql-guidance.pdf. Accessed 10 Nov 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

