Use of surrogate outcomes in US FDA drug approvals, 2003-2012: a survey
- PMID: 26614616
- PMCID: PMC4663404
- DOI: 10.1136/bmjopen-2015-007960
Use of surrogate outcomes in US FDA drug approvals, 2003-2012: a survey
Abstract
Objective: To evaluate, across a spectrum of diseases, how often surrogate outcomes are used as a basis for drug approvals by the US Food and Drug Administration (FDA), and whether and how the rationale for using treatment effects on surrogates as predictors of treatment effects on patient-centred outcomes is discussed.
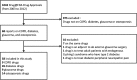
Study design and setting: We used the Drugs@FDA website to identify drug approvals produced from 2003 to 2012 by the FDA. We focused on four diseases (chronic obstructive pulmonary disease (COPD), type 1 or 2 diabetes, glaucoma and osteoporosis) for which surrogates are commonly used in trials. We reviewed the drug labels and medical reviews to provide empirical evidence on how surrogate outcomes are handled by the FDA.
Results: Of 1043 approvals screened, 58 (6%) were for the four diseases of interest. Most drugs for COPD (7/9, 78%), diabetes (26/26, 100%) and glaucoma (9/9, 100%) were approved based on surrogates while for osteoporosis, most drugs (10/14, 71%) were also approved for patient-centred outcomes (fractures). The rationale for using surrogates was discussed in 11 of the 43 (26%) drug approvals based on surrogates. In these drug approvals, we found drug approvals for diabetes are more likely than the other examined conditions to contain a discussion of trial evidence demonstrating that treatment effects on surrogate outcomes predict treatment effects on patient-centred outcomes.
Conclusions: Our results suggest that the FDA did not use a consistent approach to address surrogates in assessing the benefits and harms of drugs for COPD, type 1 or 2 diabetes, glaucoma and osteoporosis. For evaluating new drugs, patient-centred outcomes should be chosen whenever possible. If the use of surrogate outcomes is necessary, then a consistent approach is important to review the evidence for surrogacy and consider surrogate's usage in the treatment and population under study.
Keywords: EPIDEMIOLOGY; GENERAL MEDICINE (see Internal Medicine); STATISTICS & RESEARCH METHODS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources