Antihistamines for the common cold
- PMID: 26615034
- PMCID: PMC9468790
- DOI: 10.1002/14651858.CD009345.pub2
Antihistamines for the common cold
Abstract
Background: The common cold is an upper respiratory tract infection, most commonly caused by a rhinovirus. It affects people of all age groups and although in most cases it is self limiting, the common cold still causes significant morbidity. Antihistamines are commonly offered over the counter to relieve symptoms for patients affected by the common cold, however there is not much evidence of their efficacy.
Objectives: To assess the effects of antihistamines on the common cold.
Search methods: We searched CENTRAL (2015, Issue 6), MEDLINE (1948 to July week 4, 2015), EMBASE (2010 to August 2015), CINAHL (1981 to August 2015), LILACS (1982 to August 2015) and Biosis Previews (1985 to August 2015).
Selection criteria: We selected randomised controlled trials (RCTs) using antihistamines as monotherapy for the common cold. We excluded any studies with combination therapy or using antihistamines in patients with an allergic component in their illness.
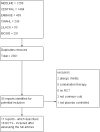
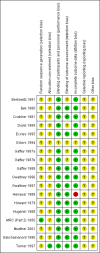
Data collection and analysis: Two authors independently assessed trial quality and extracted data. We collected adverse effects information from the included trials.
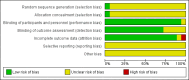
Main results: We included 18 RCTs, which were reported in 17 publications (one publication reports on two trials) with 4342 participants (of which 212 were children) suffering from the common cold, both naturally occurring and experimentally induced. The interventions consisted of an antihistamine as monotherapy compared with placebo. In adults there was a short-term beneficial effect of antihistamines on severity of overall symptoms: on day one or two of treatment 45% had a beneficial effect with antihistamines versus 38% with placebo (odds ratio (OR) 0.74, 95% confidence interval (CI) 0.60 to 0.92). However, there was no difference between antihistamines and placebo in the mid term (three to four days) to long term (six to 10 days). When evaluating individual symptoms such as nasal congestion, rhinorrhoea and sneezing, there was some beneficial effect of the sedating antihistamines compared to placebo (e.g. rhinorrhoea on day three: mean difference (MD) -0.23, 95% CI -0.39 to -0.06 on a four- or five-point severity scale; sneezing on day three: MD -0.35, 95% CI -0.49 to -0.20 on a four-point severity scale), but this effect is clinically non-significant. Adverse events such as sedation were more commonly reported with sedating antihistamines although the differences were not statistically significant. Only two trials included children and the results were conflicting. The majority of the trials had a low risk of bias although some lacked sufficient trial quality information.
Authors' conclusions: Antihistamines have a limited short-term (days one and two of treatment) beneficial effect on severity of overall symptoms but not in the mid to long term. There is no clinically significant effect on nasal obstruction, rhinorrhoea or sneezing. Although side effects are more common with sedating antihistamines, the difference is not statistically significant. There is no evidence of effectiveness of antihistamines in children.
Conflict of interest statement
An IM De Sutter: none known. Avadhesh Saraswat: none known. Mieke L van Driel: none known.
Figures











































Update of
- doi: 10.1002/14651858.CD009345
References
References to studies included in this review
Berkowitz 1991 {published data only}
-
- Berkowitz RB, Tinkelman DG. Evaluation of oral terfenadine for treatment of the common cold. Annals of Allergy 1991;67:593‐7. - PubMed
Bye 1980 {published data only}
Crutcher 1981 {published data only}
-
- Crutcher JE, Kantner TR. The effectiveness of antihistamines in the common cold. Journal of Clinical Pharmacology 1981;21:9‐15. - PubMed
Doyle 1988 {published data only}
-
- Doyle WJ, McBride TP, Skoner DP, Maddern BR, Gwaltney JM, Uhrin M. A double‐blind, placebo‐controlled clinical trial of the effect of chlorpheniramine on the response of the nasal airway, middle ear and eustachian tube to provocative rhinovirus challenge. Pediatric Infectious Disease Journal 1988;7:215‐42. - PubMed
Eccles 1995 {published data only}
-
- Eccles R, Cauwenberge P, Tetzloff W, Borum P. A clinical study to evaluate the efficacy of the antihistamine doxylamine succinate in the relief of runny nose and sneezing associated with upper respiratory tract infection. Journal of Pharmacy and Pharmacology 1995;47:990‐3. - PubMed
Ectors 1994 {published data only}
-
- Ectors L, Cauwenberghe P. Effectiveness of antihistamine cetirizine in the symptomatic treatment of rhinovirus‐induced common cold. XV Congress of European Rhinological Society and XIII International Symposium of Infection and Allergy of the Nose; 1994 June 19‐23. 1994:Abstract 265.
Gaffey 1987a {published data only}
-
- Gaffey MJ, Gwaltney JM, Sastre A, Dressler WE, Correntine JV, Hayden FG. Intranasally and orally administered antihistamine treatment of experimental rhinovirus colds. American Review of Respiratory Disease 1987;136:556‐60. - PubMed
Gaffey 1987b {published data only}
-
- Gaffey MJ, Gwaltney JM, Sastre A, Dressler WE, Correntine JV, Hayden FG. Intranasally and orally administered antihistamine treatment of experimental rhinovirus colds. American Review of Respiratory Disease 1987;136:556‐60. - PubMed
Gaffey 1988 {published and unpublished data}
-
- Gaffey MJ, Kaiser DL, Hayden FG. Ineffectiveness of oral terfenadine in natural colds: evidence against histamines a mediator of common cold symptoms. Pediatric Infectious Disease Journal 1988;7(3):215‐42. - PubMed
Gwaltney 1996 {published and unpublished data}
-
- Gwaltney JM Jr, Park J, Paul RA, Edelman DA, O'Connor RR, Turner RB. Randomized controlled trial of clemastine fumarate for treatment of experimental rhinovirus colds. Clinical Infectious Diseases 1996;22:656‐62. - PubMed
Gwaltney 1997 {published and unpublished data}
-
- Gwaltney JM, Druce HM. Efficacy of brompheniramine maleate for the treatment of rhinovirus colds. Clinical Infectious Diseases 1997;25:1188‐94. - PubMed
Henauer 1988 {published data only}
-
- Henauer SA, Glück U. Efficacy of terfenadine in the treatment of common cold. A double‐blind comparison with placebo. European Journal of Clinical Pharmacology 1988;34:35‐40. - PubMed
Howard 1979 {published data only}
-
- Howard CJ, Kantner TR, Lilienfield LS, Princiotto JV, Krum RE, Crutcher JE, et al. Effectiveness of antihistamines in the symptomatic management of the common cold. JAMA 1979;242(22):2414‐7. - PubMed
Hugenin 1988 {published data only}
-
- Hugenin M, Martin Du Pan R, Oppikofer‐Doody A‐M. Astemizole in the treatment of acute rhinopharyngitis (common cold). Double blind study in pediatrics [L'Astémizole dans le traitement de la rhinopharyngite aiguë (common cold). Etude en double aveugle en pédiatrie]. Revue Medicale de la Suisse Romande 1988;108:961‐6. - PubMed
MRC (Part 2) 1950 {published data only}
Muether 2001 {published and unpublished data}
Sakchainanont 1990 {published data only}
-
- Sakchainanont B, Chantarojanasiri T, Ruangkanchanasetr S, Tapasart C, Suwanjutha S. Effectiveness of antihistamines in common cold. Journal of the Medical Association of Thailand 1990;73(2):96‐101. - PubMed
Turner 1997 {published data only}
-
- Turner RB, Sperber SJ, Sorrentino JV, O'Connor RR, Rogers J, Batouli AR, et al. Effectiveness of clemastine fumarate for treatment of rhinorrhoea and sneezing associated with the common cold. Clinical Infectious Disease 1997;25:824‐30. - PubMed
References to studies excluded from this review
Aaronson 1968 {published data only}
-
- Aaronson AL, Ehrlich NJ, Frankel DB, Gutman AA, Aaronson DW. Effective oral nasal decongestion. A double‐blind, cross‐over analysis. Annals of Allergy 1968;26(3):145‐50. - PubMed
Andre 1974 {published data only}
-
- Andre P, Laccoureye H, Haguet F. Clinical trial of an original therapeutic agent in rhinopharyngeal diseases. Annales d'Oto‐Larangologie et de Chirurgie Cervico Faciale 1974;91(4‐5):249‐52. - PubMed
Ashe 1968 {published data only}
-
- Ashe GJ. Oral medications in nasal decongestion. A study among industrial workers. Industrial Medicine and Surgery 1968;37(3):212‐4. - PubMed
D'Agostino 1998 {published data only}
-
- D'Agostino RB Sr, Weintraub M, Russell HK, Stepanians M, D'Agostino RB Jr, Cantilena LR Jr, et al. The effectiveness of antihistamines in reducing the severity of runny nose and sneezing: a meta‐analysis. Clinical Pharmacology and Therapeutics 1998;64(6):579‐96. - PubMed
Debelic 1973 {published data only}
-
- Debelic M, Radielovic P. Therapy of banal rhinitis. Double‐blind controlled clinical study. Schweizerische Rundschau fur Medizin Praxis 1973;62(35):1074‐7. - PubMed
Dumitrescou 1965 {published data only}
-
- Dumitrescou C. Therapeutic trial in coryza. Revue Médicale de Liège 1965;20(17):465‐7. - PubMed
Elia 1967 {published data only}
-
- Elia JC. Relieving symptoms of upper respiratory tract infection in entertainers. Current Therapeutic Research, Clinical and Experimental 1967;9(9):472‐6. - PubMed
Henahan 1983 {published data only}
-
- Henahan J. No sneezing or dozing seen with new H1 blocker. JAMA 1983;249(23):3151‐2. - PubMed
Knowelden 1959 {published data only}
-
- Knowelden J. A trial of antihistaminic drugs in the treatment of the common cold. Controlled Clinical Trials: papers delivered at the conference convened by the Council for International Organizations of Medical Sciences. 1959:31‐7.
McGuinness 1976 {published data only}
-
- McGuinness BW. Trial of a long‐acting antihistamine in the treatment of coryza. British Journal of Clinical Practice 1976;30(1):15‐6. - PubMed
Shaughnessy 1999 {published data only}
-
- Shaughnessy A. Are the classic antihistamines effective in treating cold symptoms?. Evidence‐Based Practice 1999;2(4):8‐9.
Simons 1991 {published data only}
-
- Simons FE, Lukowski JL, Becker AB, Simons KJ. Comparison of the effects of single doses of the new H1‐receptor antagonists loratadine and terfenadine versus placebo in children. Journal of Pediatrics 1991;118(2):298‐300. - PubMed
Smith 1993 {published data only}
-
- Smith MBH, Feldman W. Over‐the‐counter cold medications: a critical review of clinical trials between 1950 and 1991. JAMA 1993;269(17):2258‐63. - PubMed
Tarchalska 2000 {published data only}
-
- Tarchalska BT, Zawisza EW, Niewada MN. Antihistaminic drugs in infectious rhinitis. XVII International Congress of Allergology and Clinical Immunology (ICACI); 2000, 15‐20 October; Sydney, Australia. 2000.
West 1975 {published data only}
-
- West S, Brandon B, Stolley P, Rumrill R. A review of antihistamines and the common cold. Paediatrics 1975;56(1):100‐7. - PubMed
Yoder 2006 {published data only}
-
- Yoder KE, Shaffer ML, Tournous SJ, Paul IM. Child assessment of dextromethorphan, diphenhydramine, and placebo for nocturnal cough due to upper respiratory infection. Clinical Paediatrics 2006;45(7):633‐40. - PubMed
Additional references
AlBalawi 2013
Atkins 2004
Brockwell 2001
-
- Brockwell SE, Gordon IR. A comparison of statistical methods for meta‐analysis. Statistics in Medicine 2001;20:825‐40. - PubMed
De Sutter 2012
Gonzalez 1998
-
- Gonzalez MA, Estes KS. Pharmacokinetic overview of oral second‐generation H1 antihistamines. International Journal of Clinical Pharmacology and Therapeutics 1998;36(5):292‐300. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- GRADEpro GDT. GRADEpro Guideline Development Tool [Software] [Available from www.gradepro.org]. Version August 2015. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Hayward 2012
Hemila 2013
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Innes 2006
-
- Innes JA, Reid PT. Respiratory disease. Davidson's Principles & Practice of Medicine. 20th Edition. Churchill Livingstone Elsevier, 2006:687‐9.
Jackson 1958
-
- Jackson GG, Dowling HF, Spiesman IG, Boand A. Transmission of the common cold to volunteers under controlled conditions. The common cold as clinical entity. AMA Archives of Internal Medicine 1958;101:267‐78. - PubMed
Jackson 1960
-
- Jackson GG, Dowling HF, Anderson TO. Susceptibility and immunity to common upper respiratory viral infections ‐ the common cold. Annals of Internal Medicine 1960;53:713‐38. - PubMed
Kim 2013
Langendam 2013
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Li 2013
Mann 1989
-
- Mann KV, Crowe JP, Tietze KJ. Non‐sedating histamine H1‐receptor antagonists. Clinical Pharmacy 1989;8(5):331‐44. - PubMed
Papadopoulos 2000
-
- Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, et al. Rhinoviruses infect the lower airways. Journal of Infectious Diseases 2000;181(6):1875‐84. - PubMed
Pearlman 1976
-
- Pearlman DS. Antihistamines: pharmacology and clinical use. Drugs 1976;12(4):258‐73. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rossi 2010
-
- Rossi S. Australian Medicines Handbook. 11th Edition. Adelaide: Australian Medicines Handbook Pty Ltd, 2010.
References to other published versions of this review
De Sutter 2003
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

