Enhanced optoelectronic quality of perovskite thin films with hypophosphorous acid for planar heterojunction solar cells
- PMID: 26615763
- PMCID: PMC4674686
- DOI: 10.1038/ncomms10030
Enhanced optoelectronic quality of perovskite thin films with hypophosphorous acid for planar heterojunction solar cells
Abstract
Solution-processed metal halide perovskite semiconductors, such as CH3NH3PbI3, have exhibited remarkable performance in solar cells, despite having non-negligible density of defect states. A likely candidate is halide vacancies within the perovskite crystals, or the presence of metallic lead, both generated due to the imbalanced I/Pb stoichiometry which could evolve during crystallization. Herein, we show that the addition of hypophosphorous acid (HPA) in the precursor solution can significantly improve the film quality, both electronically and topologically, and enhance the photoluminescence intensity, which leads to more efficient and reproducible photovoltaic devices. We demonstrate that the HPA can reduce the oxidized I2 back into I(-), and our results indicate that this facilitates an improved stoichiometry in the perovskite crystal and a reduced density of metallic lead.
Figures
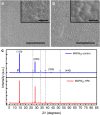
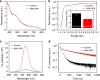
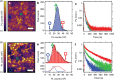

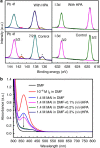
References
-
- Kojima A., Teshima K., Shirai Y. & Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009). - PubMed
-
- Lee M. M., Teuscher J., Miyasaka T., Murakami T. N. & Snaith H. J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 338, 643–647 (2012). - PubMed
-
- Tan Z.-K. et al.. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 9, 687–692 (2014). - PubMed
-
- Yang W. S. et al.. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348, 1234–1237 (2015). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

