Single-molecule spectroscopy and imaging over the decades
- PMID: 26616210
- PMCID: PMC4782608
- DOI: 10.1039/c5fd00149h
Single-molecule spectroscopy and imaging over the decades
Abstract
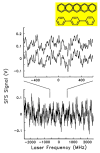
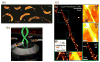
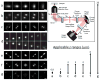
As of 2015, it has been 26 years since the first optical detection and spectroscopy of single molecules in condensed matter. This area of science has expanded far beyond the early low temperature studies in crystals to include single molecules in cells, polymers, and in solution. The early steps relied upon high-resolution spectroscopy of inhomogeneously broadened optical absorption profiles of molecular impurities in solids at low temperatures. Spectral fine structure arising directly from the position-dependent fluctuations of the number of molecules in resonance led to the attainment of the single-molecule limit in 1989 using frequency-modulation laser spectroscopy. In the early 1990s, a variety of fascinating physical effects were observed for individual molecules, including imaging of the light from single molecules as well as observations of spectral diffusion, optical switching and the ability to select different single molecules in the same focal volume simply by tuning the pumping laser frequency. In the room temperature regime, researchers showed that bursts of light from single molecules could be detected in solution, leading to imaging and microscopy by a variety of methods. Studies of single copies of the green fluorescent protein also uncovered surprises, especially the blinking and photoinduced recovery of emitters, which stimulated further development of photoswitchable fluorescent protein labels. All of these early steps provided important fundamentals underpinning the development of super-resolution microscopy based on single-molecule localization and active control of emitting concentration. Current thrust areas include extensions to three-dimensional imaging with high precision, orientational analysis of single molecules, and direct measurements of photodynamics and transport properties for single molecules trapped in solution by suppression of Brownian motion. Without question, a huge variety of studies of single molecules performed by many talented scientists all over the world have extended our knowledge of the nanoscale and many microscopic mechanisms previously hidden by ensemble averaging.
Figures
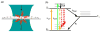






References
-
- Moerner WE, Kador L. Phys Rev Lett. 1989;62:2535–2538. - PubMed
-
- Moerner WE, Basché T. Angew Chem Int Ed. 1993;105:537.
-
- Moerner WE. Science. 1994;265:46–53. - PubMed
-
- Orrit M, Bernard J, Brown R, Lounis B. Prog Optics. 1996;35:61–144.
-
- Plakhotnik T, Donley EA, Wild UP. Annu Rev Phys Chem. 1997;48:181–212. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

