Inhibition of insulin fibrillation by osmolytes: Mechanistic insights
- PMID: 26616401
- PMCID: PMC4663473
- DOI: 10.1038/srep17599
Inhibition of insulin fibrillation by osmolytes: Mechanistic insights
Abstract
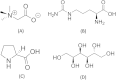
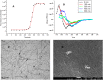
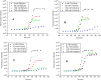
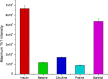
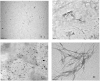
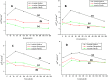
We have studied here using a number of biophysical tools the effects of osmolytes, betaine, citrulline, proline and sorbitol which differ significantly in terms of their physical characteristics such as, charge distribution, polarity, H-bonding abilities etc, on the fibrillation of insulin. Among these, betaine, citrulline, and proline are very effective in decreasing the extent of fibrillation. Proline also causes a substantial delay in the onset of fibrillation in the concentration range (50-250 mM) whereas such an effect is seen for citrulline only at 250 mM, and in case of betaine this effect is not seen at all in the whole concentration range. The enthalpies of interaction at various stages of fibrillation process have suggested that the preferential exclusion of the osmolyte and its polar interaction with the protein are important in inhibition. The results indicate that the osmolytes are most effective when added prior to the elongation stage of fibrillation. These observations have significant biological implications, since insulin fibrillation is known to cause injection amyloidosis and our data may help in designing lead drug molecules and development of potential therapeutic strategies.
Figures

Similar articles
-
Selective inhibition of aggregation/fibrillation of bovine serum albumin by osmolytes: Mechanistic and energetics insights.PLoS One. 2017 Feb 16;12(2):e0172208. doi: 10.1371/journal.pone.0172208. eCollection 2017. PLoS One. 2017. PMID: 28207877 Free PMC article.
-
Osmolyte effects on the self-association of concanavalin A: testing theoretical models.Biochemistry. 2013 Dec 23;52(51):9367-74. doi: 10.1021/bi401049s. Epub 2013 Nov 22. Biochemistry. 2013. PMID: 24215492
-
Osmolyte controlled fibrillation kinetics of insulin: New insight into fibrillation using the preferential exclusion principle.Biotechnol Prog. 2009 Sep-Oct;25(5):1508-14. doi: 10.1002/btpr.255. Biotechnol Prog. 2009. PMID: 19653270
-
The physical chemistry of the amyloid phenomenon: thermodynamics and kinetics of filamentous protein aggregation.Essays Biochem. 2014;56:11-39. doi: 10.1042/bse0560011. Essays Biochem. 2014. PMID: 25131584 Review.
-
[Osmolytes and osmoregulation in renal cells].Postepy Biochem. 1996;42(2):167-77. Postepy Biochem. 1996. PMID: 8805191 Review. Polish. No abstract available.
Cited by
-
Osmolytes and crowders regulate aggregation of the cancer-related L106R mutant of the Axin protein.Biophys J. 2021 Aug 17;120(16):3455-3469. doi: 10.1016/j.bpj.2021.05.024. Epub 2021 Jun 2. Biophys J. 2021. PMID: 34087214 Free PMC article.
-
A Small Molecule Impedes Insulin Fibrillation: Another New Role of Phenothiazine Derivatives.ChemistryOpen. 2017 Dec 7;7(1):68-79. doi: 10.1002/open.201700131. eCollection 2018 Jan. ChemistryOpen. 2017. PMID: 29318099 Free PMC article.
-
Trehalose Effect on the Aggregation of Model Proteins into Amyloid Fibrils.Life (Basel). 2020 May 13;10(5):60. doi: 10.3390/life10050060. Life (Basel). 2020. PMID: 32414105 Free PMC article.
-
Selective inhibition of aggregation/fibrillation of bovine serum albumin by osmolytes: Mechanistic and energetics insights.PLoS One. 2017 Feb 16;12(2):e0172208. doi: 10.1371/journal.pone.0172208. eCollection 2017. PLoS One. 2017. PMID: 28207877 Free PMC article.
-
Inhibition of Insulin Amyloid Fibrillation by a Novel Amphipathic Heptapeptide: MECHANISTIC DETAILS STUDIED BY SPECTROSCOPY IN COMBINATION WITH MICROSCOPY.J Biol Chem. 2016 Nov 4;291(45):23545-23556. doi: 10.1074/jbc.M116.742460. Epub 2016 Sep 27. J Biol Chem. 2016. PMID: 27679488 Free PMC article.
References
-
- Chiti F. & Dobson C. M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 (2006). - PubMed
-
- Herczenik E. & Gebbink M. F. Molecular and cellular aspects of protein misfolding and disease. FASEB J. 22, 2115–2133 (2008). - PubMed
-
- Sunde M. et al. The common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Bio. 273, 729–739 (1997). - PubMed
-
- McLaurin J., Kierstead M. & Fraser P. Anti-beta amyloid aggregation therapy. Neurobiol. Aging, 23, 1580–1586 (2002).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

