Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease
- PMID: 26616834
- PMCID: PMC4663725
- DOI: 10.1186/s13059-015-0826-7
Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease
Abstract
Background: The RNA-guided Cas9 system represents a flexible approach for genome editing in plants. This method can create specific mutations that knock-out or alter target gene function. It provides a valuable tool for plant research and offers opportunities for crop improvement.
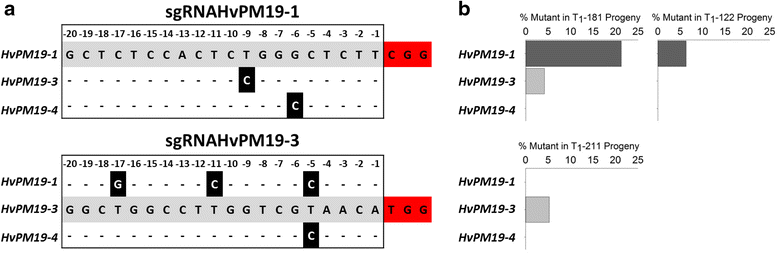
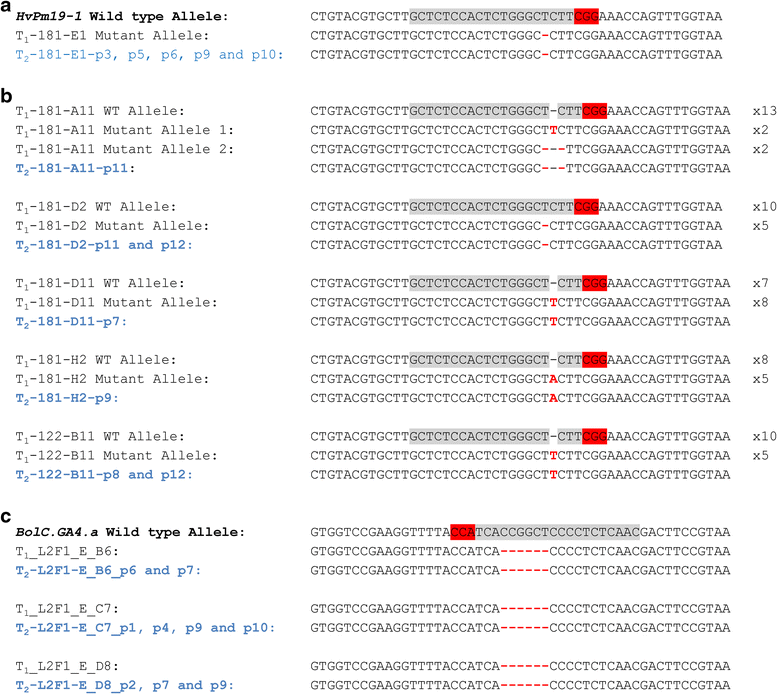
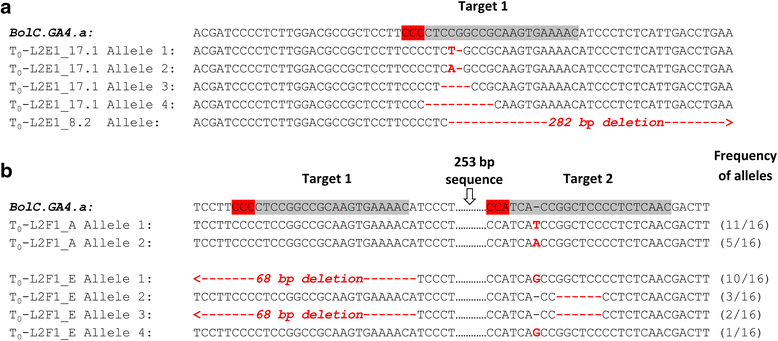
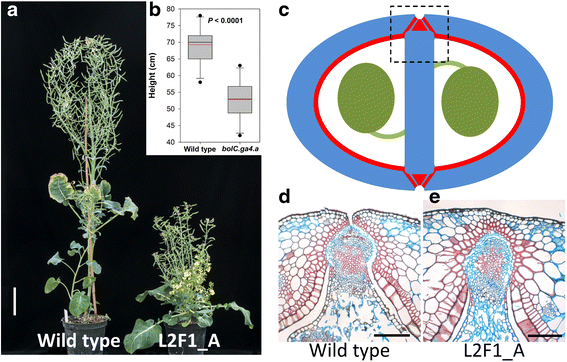
Results: We investigate the use and target specificity requirements of RNA-guided Cas9 genome editing in barley (Hordeum vulgare) and Brassica oleracea by targeting multicopy genes. In barley, we target two copies of HvPM19 and observe Cas9-induced mutations in the first generation of 23 % and 10 % of the lines, respectively. In B. oleracea, targeting of BolC.GA4.a leads to Cas9-induced mutations in 10 % of first generation plants screened. In addition, a phenotypic screen identifies T0 plants with the expected dwarf phenotype associated with knock-out of the target gene. In both barley and B. oleracea stable Cas9-induced mutations are transmitted to T2 plants independently of the T-DNA construct. We observe off-target activity in both species, despite the presence of at least one mismatch between the single guide RNA and the non-target gene sequences. In barley, a transgene-free plant has concurrent mutations in the target and non-target copies of HvPM19.
Conclusions: We demonstrate the use of RNA-guided Cas9 to generate mutations in target genes of both barley and B. oleracea and show stable transmission of these mutations thus establishing the potential for rapid characterisation of gene function in these species. In addition, the off-target effects reported offer both potential difficulties and specific opportunities to target members of multigene families in crops.
Figures



References
Publication types
MeSH terms
Grants and funding
- BBS/E/J/00000613/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/J/000C0628/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L014130/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004553/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

