CCR6(+) Th cell populations distinguish ACPA positive from ACPA negative rheumatoid arthritis
- PMID: 26617177
- PMCID: PMC4663738
- DOI: 10.1186/s13075-015-0800-5
CCR6(+) Th cell populations distinguish ACPA positive from ACPA negative rheumatoid arthritis
Abstract
Introduction: Patients with rheumatoid arthritis (RA) can be separated into two major subpopulations based on the absence or presence of serum anti-citrullinated protein antibodies (ACPAs). The more severe disease course in ACPA(+) RA and differences in treatment outcome between these subpopulations suggest that ACPA(+) and ACPA(-) RA are different disease subsets. The identification of T-helper (Th) cells specifically recognizing citrullinated peptides, combined with the strong association between HLA-DRB1 and ACPA positivity, point toward a pathogenic role of Th cells in ACPA(+) RA. In this context we recently identified a potential pathogenic role for CCR6(+) Th cells in RA. Therefore, we examined whether Th cell population distributions differ by ACPA status.
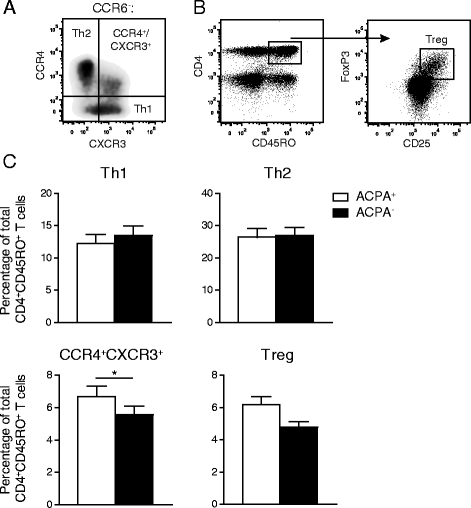
Methods: We performed a nested matched case-control study including 27 ACPA(+) and 27 ACPA(-) treatment-naive early RA patients matched for disease activity score in 44 joints, presence of rheumatoid factor, sex, age, duration of complaints and presence of erosions. CD4(+)CD45RO(+) (memory) Th cell distribution profiles from these patients were generated based on differential chemokine receptor expression and related with disease duration.
Results: ACPA status was not related to differences in total CD4(+) T cell or memory Th cell proportions. However, ACPA(+) patients had significantly higher proportions of Th cells expressing the chemokine receptors CCR6 and CXCR3. Similar proportions of CCR4(+) and CCR10(+) Th cells were found. Within the CCR6(+) cell population, four Th subpopulations were distinguished based on differential chemokine receptor expression: Th17 (CCR4(+)CCR10(-)), Th17.1 (CXCR3(+)), Th22 (CCR4(+)CCR10(+)) and CCR4/CXCR3 double-positive (DP) cells. In particular, higher proportions of Th22 (p = 0.02), Th17.1 (p = 0.03) and CCR4/CXCR3 DP (p = 0.01) cells were present in ACPA(+) patients. In contrast, ACPA status was not associated with differences in Th1 (CCR6(-)CXCR3(+); p = 0.90), Th2 (CCR6(-)CCR4(+); p = 0.27) and T-regulatory (CD25(hi)FOXP3(+); p = 0.06) cell proportions. Interestingly, CCR6(+) Th cells were inversely correlated with disease duration in ACPA(-) patients (R(2) = -0.35; p < 0.01) but not in ACPA(+) (R(2) < 0.01; p = 0.94) patients.
Conclusions: These findings demonstrate that increased peripheral blood CCR6(+) Th cells proportions distinguish ACPA(+) RA from ACPA(-) RA. This suggests that CCR6(+) Th cells are involved in the differences in disease severity and treatment outcome between ACPA(+) and ACPA(-) RA.
Figures



Similar articles
-
Circulating T helper and T regulatory subsets in untreated early rheumatoid arthritis and healthy control subjects.J Leukoc Biol. 2016 Oct;100(4):823-833. doi: 10.1189/jlb.5A0116-025R. Epub 2016 May 17. J Leukoc Biol. 2016. PMID: 27190305
-
Follicular helper T cells in peripheral blood of patients with rheumatoid arthritis.Reumatol Clin. 2017 Nov-Dec;13(6):338-343. doi: 10.1016/j.reuma.2016.07.003. Epub 2016 Aug 29. Reumatol Clin. 2017. PMID: 27595364 English, Spanish.
-
Chemokine receptor co-expression reveals aberrantly distributed TH effector memory cells in GPA patients.Arthritis Res Ther. 2017 Jun 14;19(1):136. doi: 10.1186/s13075-017-1343-8. Arthritis Res Ther. 2017. PMID: 28615072 Free PMC article.
-
The role and modulation of CCR6+ Th17 cell populations in rheumatoid arthritis.Cytokine. 2015 Jul;74(1):43-53. doi: 10.1016/j.cyto.2015.02.002. Epub 2015 Mar 28. Cytokine. 2015. PMID: 25828206 Review.
-
Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation-Lessons From Rheumatoid Arthritis.Front Immunol. 2019 Mar 12;10:353. doi: 10.3389/fimmu.2019.00353. eCollection 2019. Front Immunol. 2019. PMID: 30915067 Free PMC article. Review.
Cited by
-
T helper cells in synovial fluid of patients with rheumatoid arthritis primarily have a Th1 and a CXCR3+Th2 phenotype.Arthritis Res Ther. 2020 Oct 16;22(1):245. doi: 10.1186/s13075-020-02349-y. Arthritis Res Ther. 2020. PMID: 33066816 Free PMC article.
-
Regulatory and Effector Cell Disequilibrium in Patients with Acute Cellular Rejection and Chronic Lung Allograft Dysfunction after Lung Transplantation: Comparison of Peripheral and Alveolar Distribution.Cells. 2021 Apr 1;10(4):780. doi: 10.3390/cells10040780. Cells. 2021. PMID: 33916034 Free PMC article.
-
Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview.Front Immunol. 2021 Feb 22;12:637829. doi: 10.3389/fimmu.2021.637829. eCollection 2021. Front Immunol. 2021. PMID: 33692806 Free PMC article. Review.
-
The Effect of PEGylated Graphene Oxide Nanoparticles on the Th17-Polarization of Activated T Helpers.Materials (Basel). 2023 Jan 16;16(2):877. doi: 10.3390/ma16020877. Materials (Basel). 2023. PMID: 36676614 Free PMC article.
-
ACPA Status Correlates with Differential Immune Profile in Patients with Rheumatoid Arthritis.Cells. 2021 Mar 14;10(3):647. doi: 10.3390/cells10030647. Cells. 2021. PMID: 33799480 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

