Elevated Temperature Differentially Influences Effector-Triggered Immunity Outputs in Arabidopsis
- PMID: 26617631
- PMCID: PMC4637416
- DOI: 10.3389/fpls.2015.00995
Elevated Temperature Differentially Influences Effector-Triggered Immunity Outputs in Arabidopsis
Abstract
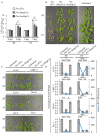
Pseudomonas syringae is a Gram-negative bacterium that infects multiple plant species by manipulating cellular processes via injection of type three secreted effectors (T3SEs) into host cells. Nucleotide-binding leucine-rich repeat (NLR) resistance (R) proteins recognize specific T3SEs and trigger a robust immune response, called effector-triggered immunity (ETI), which limits pathogen proliferation and is often associated with localized programmed cell death, known as the hypersensitive response (HR). In this study, we examine the influence of elevated temperature on two ETI outputs: HR and pathogen virulence suppression. We found that in the Arabidopsis thaliana accession Col-0, elevated temperatures suppress the HR, but have minimal influence on ETI-associated P. syringae virulence suppression, thereby uncoupling these two ETI responses. We also identify accessions of Arabidopsis that exhibit impaired P. syringae virulence suppression at elevated temperature, highlighting the natural variation that exists in coping with biotic and abiotic stresses. These results not only reinforce the influence of abiotic factors on plant immunity but also emphasize the importance of carefully documented environmental conditions in studies of plant immunity.
Keywords: Arabidopsis thaliana; Pseudomonas syringae; abiotic stress; disease resistance; effector-triggered immunity; elevated temperature; hypersensitive response; programmed cell death.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

