Lipopolysaccharide induces a downregulation of adiponectin receptors in-vitro and in-vivo
- PMID: 26618091
- PMCID: PMC4655095
- DOI: 10.7717/peerj.1428
Lipopolysaccharide induces a downregulation of adiponectin receptors in-vitro and in-vivo
Abstract
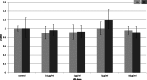
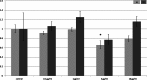
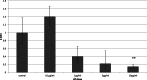
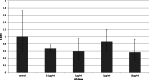
Background. Adipose tissue contributes to the inflammatory response through production of cytokines, recruitment of macrophages and modulation of the adiponectin system. Previous studies have identified a down-regulation of adiponectin in pathologies characterised by acute (sepsis and endotoxaemia) and chronic inflammation (obesity and type-II diabetes mellitus). In this study, we investigated the hypothesis that LPS would reduce adiponectin receptor expression in a murine model of endotoxaemia and in adipoocyte and myocyte cell cultures. Methods. 25 mg/kg LPS was injected intra-peritoneally into C57BL/6J mice, equivalent volumes of normal saline were used in control animals. Mice were killed at 4 or 24 h post injection and tissues harvested. Murine adipocytes (3T3-L1) and myocytes (C2C12) were grown in standard culture, treated with LPS (0.1 µg/ml-10 µg/ml) and harvested at 4 and 24 h. RNA was extracted and qPCR was conducted according to standard protocols and relative expression was calculated. Results. After LPS treatment there was a significant reduction after 4 h in gene expression of adipo R1 in muscle and peri-renal fat and of adipo R2 in liver, peri-renal fat and abdominal wall subcutaneous fat. After 24 h, significant reductions were limited to muscle. Cell culture extracts showed varied changes with reduction in adiponectin and adipo R2 gene expression only in adipocytes. Conclusions. LPS reduced adiponectin receptor gene expression in several tissues including adipocytes. This reflects a down-regulation of this anti-inflammatory and insulin-sensitising pathway in response to LPS. The trend towards base line after 24 h in tissue depots may reflect counter-regulatory mechanisms. Adiponectin receptor regulation differs in the tissues investigated.
Keywords: Adiponectin; Adiponectin receptors; Adipose tissue; Lipopolysaccharide.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

