Interactions of conjugate vaccines and co-administered vaccines
- PMID: 26619353
- PMCID: PMC4962715
- DOI: 10.1080/21645515.2015.1091908
Interactions of conjugate vaccines and co-administered vaccines
Abstract
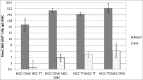
Conjugate vaccines play an important role in the prevention of infectious diseases such as those caused by the bacteria Haemophilus influenzae (Hi) type b (Hib), Neisseria meningitidis, and Streptococcus pneumoniae. Vaccines developed against these 3 pathogens utilize 3 main carrier proteins, non-toxic mutant of diphtheria toxin (CRM197), diphtheria toxoid (DT) and tetanus toxoid (TT). Current pediatric immunisation schedules include the administration of several vaccines simultaneously, therefore increasing the potential for immune interference (both positively and negatively) to the antigens administered. Knowledge of vaccine interactions is principally derived from clinical trials, these are reviewed here to explore immune interference which may result of from carrier-specific T-cell helper interactions, bystander interference and carrier induced epitopic suppression.
Keywords: Vaccines; conjugate; immunisation; infectious disease; interactions; pediatric.
Figures
References
-
- Dagan R, Poolman JT, Zepp F. Combination vaccines containing DTPa-Hib: impact of IPV and coadministration of CRM197 conjugates. Expert Rev Vaccines 2008; 7:97-115; PMID:18251697; http://dx.doi.org/10.1586/14760584.7.1.97 - DOI - PubMed
-
- Miller E, Andrews N, Waight P, Findlow H, Ashton L, England A, Stanford E, Matheson M, Southern J, Sheasby E, et al.. Safety and immunogenicity of coadministering a combined meningococcal serogroup C and Haemophilus influenzae type b conjugate vaccine with 7-valent pneumococcal conjugate vaccine and measles, mumps, and rubella vaccine at 12 months of age. Clin Vaccine Immunol 2011; 18(3):367-72; PMID:21191076; http://dx.doi.org/10.1128/CVI.00516-10 - DOI - PMC - PubMed
-
- Kshirsagar N, Mur N, Thatte U, Gogtay N, Viviani S, Preziosi M-P, Elie C, Findlow H, Carlone G, Borrow R, et al.. Safety and immunogenicity of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine 2007; 25S:A101-7; http://dx.doi.org/10.1016/j.vaccine.2007.04.050 - DOI - PubMed
-
- Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: A review. Vaccine 2010; 28(34):5513-23; PMID:20600514; http://dx.doi.org/10.1016/j.vaccine.2010.06.026 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
