Acetylation of glucokinase regulatory protein decreases glucose metabolism by suppressing glucokinase activity
- PMID: 26620281
- PMCID: PMC4664969
- DOI: 10.1038/srep17395
Acetylation of glucokinase regulatory protein decreases glucose metabolism by suppressing glucokinase activity
Abstract
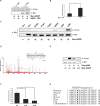
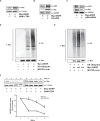
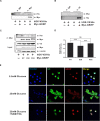
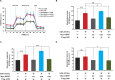
Glucokinase (GK), mainly expressed in the liver and pancreatic β-cells, is critical for maintaining glucose homeostasis. GK expression and kinase activity, respectively, are both modulated at the transcriptional and post-translational levels. Post-translationally, GK is regulated by binding the glucokinase regulatory protein (GKRP), resulting in GK retention in the nucleus and its inability to participate in cytosolic glycolysis. Although hepatic GKRP is known to be regulated by allosteric mechanisms, the precise details of modulation of GKRP activity, by post-translational modification, are not well known. Here, we demonstrate that GKRP is acetylated at Lys5 by the acetyltransferase p300. Acetylated GKRP is resistant to degradation by the ubiquitin-dependent proteasome pathway, suggesting that acetylation increases GKRP stability and binding to GK, further inhibiting GK nuclear export. Deacetylation of GKRP is effected by the NAD(+)-dependent, class III histone deacetylase SIRT2, which is inhibited by nicotinamide. Moreover, the livers of db/db obese, diabetic mice also show elevated GKRP acetylation, suggesting a broader, critical role in regulating blood glucose. Given that acetylated GKRP may affiliate with type-2 diabetes mellitus (T2DM), understanding the mechanism of GKRP acetylation in the liver could reveal novel targets within the GK-GKRP pathway, for treating T2DM and other metabolic pathologies.
Figures

Similar articles
-
Molecular basis for the role of glucokinase regulatory protein as the allosteric switch for glucokinase.Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):10171-6. doi: 10.1073/pnas.1300457110. Epub 2013 Jun 3. Proc Natl Acad Sci U S A. 2013. PMID: 23733961 Free PMC article.
-
Sirt2 facilitates hepatic glucose uptake by deacetylating glucokinase regulatory protein.Nat Commun. 2018 Jan 2;9(1):30. doi: 10.1038/s41467-017-02537-6. Nat Commun. 2018. PMID: 29296001 Free PMC article.
-
An allosteric activator of glucokinase impairs the interaction of glucokinase and glucokinase regulatory protein and regulates glucose metabolism.J Biol Chem. 2006 Dec 8;281(49):37668-74. doi: 10.1074/jbc.M605186200. Epub 2006 Oct 6. J Biol Chem. 2006. PMID: 17028192
-
Hormonal and Metabolite Regulation of Hepatic Glucokinase.Annu Rev Nutr. 2016 Jul 17;36:389-415. doi: 10.1146/annurev-nutr-071715-051145. Epub 2016 May 4. Annu Rev Nutr. 2016. PMID: 27146014 Review.
-
Molecular targeting of the GK-GKRP pathway in diabetes.Expert Opin Ther Targets. 2015 Jan;19(1):129-39. doi: 10.1517/14728222.2014.965681. Epub 2014 Oct 17. Expert Opin Ther Targets. 2015. PMID: 25324018 Review.
Cited by
-
L-Arginine prevents cereblon-mediated ubiquitination of glucokinase and stimulates glucose-6-phosphate production in pancreatic β-cells.Commun Biol. 2020 Sep 8;3(1):497. doi: 10.1038/s42003-020-01226-3. Commun Biol. 2020. PMID: 32901087 Free PMC article.
-
Hepatic Transcriptome Analysis Revealing the Molecular Pathogenesis of Type 2 Diabetes Mellitus in Zucker Diabetic Fatty Rats.Front Endocrinol (Lausanne). 2020 Nov 24;11:565858. doi: 10.3389/fendo.2020.565858. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33329383 Free PMC article.
-
GCKR Polymorphisms Increase the Risks of Low Bone Mineral Density in Young and Non-Obese Patients With MASLD and Hyperuricemia.Kaohsiung J Med Sci. 2025 Jun;41(6):e70017. doi: 10.1002/kjm2.70017. Epub 2025 Apr 9. Kaohsiung J Med Sci. 2025. PMID: 40202351 Free PMC article.
-
Exploring histone deacetylases in type 2 diabetes mellitus: pathophysiological insights and therapeutic avenues.Clin Epigenetics. 2024 Jun 11;16(1):78. doi: 10.1186/s13148-024-01692-0. Clin Epigenetics. 2024. PMID: 38862980 Free PMC article. Review.
-
Roles of KLF4 and AMPK in the inhibition of glycolysis by pulsatile shear stress in endothelial cells.Proc Natl Acad Sci U S A. 2021 May 25;118(21):e2103982118. doi: 10.1073/pnas.2103982118. Proc Natl Acad Sci U S A. 2021. PMID: 34001623 Free PMC article.
References
-
- Mokdad A. H. et al.. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289, 76–79 (2003). - PubMed
-
- Consoli A. Role of liver in pathophysiology of NIDDM. Diabetes Care 15, 430–441 (1992). - PubMed
-
- Matschinsky F. M. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes 39, 647–652 (1990). - PubMed
-
- Nordlie R. C., Foster J. D. & Lange A. J. Regulation of glucose production by the liver. Annu Rev Nutr 19, 379–406 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

