Effect of Age and Refractive Error on the Melanopsin Mediated Post-Illumination Pupil Response (PIPR)
- PMID: 26620343
- PMCID: PMC4664956
- DOI: 10.1038/srep17610
Effect of Age and Refractive Error on the Melanopsin Mediated Post-Illumination Pupil Response (PIPR)
Abstract
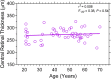
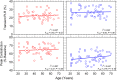
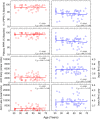
Melanopsin containing intrinsically photosensitive Retinal Ganglion cells (ipRGCs) mediate the pupil light reflex (PLR) during light onset and at light offset (the post-illumination pupil response, PIPR). Recent evidence shows that the PLR and PIPR can provide non-invasive, objective markers of age-related retinal and optic nerve disease; however there is no consensus on the effects of healthy ageing or refractive error on the ipRGC mediated pupil function. Here we isolated melanopsin contributions to the pupil control pathway in 59 human participants with no ocular pathology across a range of ages and refractive errors. We show that there is no effect of age or refractive error on ipRGC inputs to the human pupil control pathway. The stability of the ipRGC mediated pupil response across the human lifespan provides a functional correlate of their robustness observed during ageing in rodent models.
Figures



References
-
- Dacey D. M. et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, 749–754 (2005). - PubMed
-
- Berson D. M., Dunn F. A. & Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

