Nitro-oleic acid modulates classical and regulatory activation of macrophages and their involvement in pro-fibrotic responses
- PMID: 26620549
- PMCID: PMC4748956
- DOI: 10.1016/j.freeradbiomed.2015.11.026
Nitro-oleic acid modulates classical and regulatory activation of macrophages and their involvement in pro-fibrotic responses
Abstract
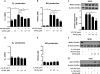
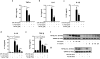
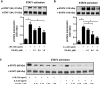
Inflammation is an immune response triggered by microbial invasion and/or tissue injury. While acute inflammation is directed toward invading pathogens and injured cells, thus enabling tissue regeneration, chronic inflammation can lead to severe pathologies and tissue dysfunction. These processes are linked with macrophage polarization into specific inflammatory "M1-like" or regulatory "M2-like" subsets. Nitro-fatty acids (NO2-FAs), produced endogenously as byproducts of metabolism and oxidative inflammatory conditions, may be useful for treating diseases associated with dysregulated immune homeostasis. The goal of this study was to characterize the role of nitro-oleic acid (OA-NO2) in regulating the functional specialization of macrophages induced by bacterial lipopolysaccharide or interleukin-4, and to reveal specific signaling mechanisms which can account for OA-NO2-dependent modulation of inflammation and fibrotic responses. Our results show that OA-NO2 inhibits lipopolysaccharide-stimulated production of both pro-inflammatory and immunoregulatory cytokines (including transforming growth factor-β) and inhibits nitric oxide and superoxide anion production. OA-NO2 also decreases interleukin-4-induced macrophage responses by inhibiting arginase-I expression and transforming growth factor-β production. These effects are mediated via downregulation of signal transducers and activators of transcription, mitogen-activated protein kinase and nuclear factor-кB signaling responses. Finally, OA-NO2 inhibits fibrotic processes in an in vivo model of angiotensin II-induced myocardial fibrosis by attenuating expression of α-smooth muscle actin, systemic transforming growth factor-β levels and infiltration of both "M1-" and "M2-like" macrophage subsets into afflicted tissue. Overall, the electrophilic fatty acid derivative OA-NO2 modulates a broad range of "M1-" and "M2-like" macrophage functions and represents a potential therapeutic approach to target diseases associated with dysregulated macrophage subsets.
Keywords: Fibrosis; Inflammation; Macrophage functional specialization; Macrophages; Nitro-fatty acids; Nitro-oleic acid.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

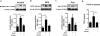
References
-
- Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunologic research. 2012;53:11–24. - PubMed
-
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nature reviews. Immunology. 2011;11:750–761. - PubMed
-
- Ohmori Y, Hamilton TA. Requirement for STAT1 in LPS-induced gene expression in macrophages. Journal of leukocyte biology. 2001;69:598–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

