Transmission of HIV Drug Resistance and the Predicted Effect on Current First-line Regimens in Europe
- PMID: 26620652
- PMCID: PMC4741360
- DOI: 10.1093/cid/civ963
Transmission of HIV Drug Resistance and the Predicted Effect on Current First-line Regimens in Europe
Abstract
Background: Numerous studies have shown that baseline drug resistance patterns may influence the outcome of antiretroviral therapy. Therefore, guidelines recommend drug resistance testing to guide the choice of initial regimen. In addition to optimizing individual patient management, these baseline resistance data enable transmitted drug resistance (TDR) to be surveyed for public health purposes. The SPREAD program systematically collects data to gain insight into TDR occurring in Europe since 2001.
Methods: Demographic, clinical, and virological data from 4140 antiretroviral-naive human immunodeficiency virus (HIV)-infected individuals from 26 countries who were newly diagnosed between 2008 and 2010 were analyzed. Evidence of TDR was defined using the WHO list for surveillance of drug resistance mutations. Prevalence of TDR was assessed over time by comparing the results to SPREAD data from 2002 to 2007. Baseline susceptibility to antiretroviral drugs was predicted using the Stanford HIVdb program version 7.0.
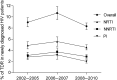
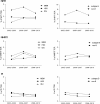
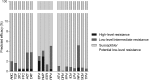
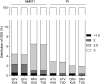
Results: The overall prevalence of TDR did not change significantly over time and was 8.3% (95% confidence interval, 7.2%-9.5%) in 2008-2010. The most frequent indicators of TDR were nucleoside reverse transcriptase inhibitor (NRTI) mutations (4.5%), followed by nonnucleoside reverse transcriptase inhibitor (NNRTI) mutations (2.9%) and protease inhibitor mutations (2.0%). Baseline mutations were most predictive of reduced susceptibility to initial NNRTI-based regimens: 4.5% and 6.5% of patient isolates were predicted to have resistance to regimens containing efavirenz or rilpivirine, respectively, independent of current NRTI backbones.
Conclusions: Although TDR was highest for NRTIs, the impact of baseline drug resistance patterns on susceptibility was largest for NNRTIs. The prevalence of TDR assessed by epidemiological surveys does not clearly indicate to what degree susceptibility to different drug classes is affected.
Keywords: Europe; HIV-1; antiretroviral therapy; drug resistance; transmission.
© The Author 2015. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Little SJ, Holte S, Routy JP et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med 2002; 347:385–94. - PubMed
-
- Violin M, Cozzi-Lepri A, Velleca R et al. Risk of failure in patients with 215 HIV-1 revertants starting their first thymidine analog-containing highly active antiretroviral therapy. AIDS 2004; 18:227–35. - PubMed
-
- Kuritzkes DR, Lalama CM, Ribaudo HJ et al. Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J Infect Dis 2008; 197:867–70. - PubMed
-
- Wittkop L, Gunthard HF, de Wolf F et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis 2011; 11:363–71. - PubMed
-
- European AIDS Clinical Society. EACS guidelines version 8.0. Available at: http://www.eacsociety.org/files/guidelines_8_0-english_web.pdf. Accessed 29 Octobre 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

