A biomimetic hybrid nanoplatform for encapsulation and precisely controlled delivery of theranostic agents. [Corrected]
- PMID: 26621191
- PMCID: PMC4686774
- DOI: 10.1038/ncomms10081
A biomimetic hybrid nanoplatform for encapsulation and precisely controlled delivery of theranostic agents. [Corrected]
Erratum in
-
Erratum: A biomimetic hybrid nanoplatform for encapsulation and precisely controlled delivery of theranostic agents.Nat Commun. 2016 Jan 7;7:10350. doi: 10.1038/ncomms10350. Nat Commun. 2016. PMID: 26739909 Free PMC article. No abstract available.
Abstract
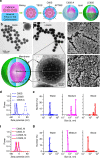
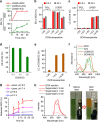
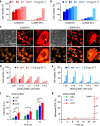
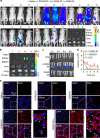
Nanoparticles have demonstrated great potential for enhancing drug delivery. However, the low drug encapsulation efficiency at high drug-to-nanoparticle feeding ratios and minimal drug loading content in nanoparticle at any feeding ratios are major hurdles to their widespread applications. Here we report a robust eukaryotic cell-like hybrid nanoplatform (EukaCell) for encapsulation of theranostic agents (doxorubicin and indocyanine green). The EukaCell consists of a phospholipid membrane, a cytoskeleton-like mesoporous silica matrix and a nucleus-like fullerene core. At high drug-to-nanoparticle feeding ratios (for example, 1:0.5), the encapsulation efficiency and loading content can be improved by 58 and 21 times, respectively, compared with conventional silica nanoparticles. Moreover, release of the encapsulated drug can be precisely controlled via dosing near infrared laser irradiation. Ultimately, the ultra-high (up to ∼87%) loading content renders augmented anticancer capacity both in vitro and in vivo. Our EukaCell is valuable for drug delivery to fight against cancer and potentially other diseases.
Conflict of interest statement
The first and corresponding authors disclosed the idea in this work to the Technology and Commercialization Office at the Ohio State University. The other authors declare no competing financial interests.
Figures
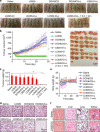
References
-
- Fisher B. et al.. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 16, 2672–2685 (1998). - PubMed
-
- Coates A. et al.. On the receiving end patient perception of the side-effects of cancer-chemotherapy. Eur. J. Cancer Clin. Oncol. 19, 203–208 (1983). - PubMed
-
- Frechet J. M. Functional polymers and dendrimers - reactivity, molecular architecture, and interfacial energy. Science 263, 1710–1715 (1994). - PubMed
-
- Sinha R., Kim G. J., Nie S. M. & Shin D. M. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 5, 1909–1917 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

