Reversion to an embryonic alternative splicing program enhances leukemia stem cell self-renewal
- PMID: 26621726
- PMCID: PMC4687548
- DOI: 10.1073/pnas.1506943112
Reversion to an embryonic alternative splicing program enhances leukemia stem cell self-renewal
Abstract
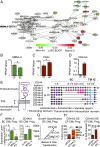
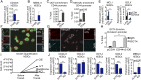
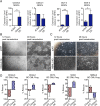
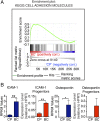
Formative research suggests that a human embryonic stem cell-specific alternative splicing gene regulatory network, which is repressed by Muscleblind-like (MBNL) RNA binding proteins, is involved in cell reprogramming. In this study, RNA sequencing, splice isoform-specific quantitative RT-PCR, lentiviral transduction, and in vivo humanized mouse model studies demonstrated that malignant reprogramming of progenitors into self-renewing blast crisis chronic myeloid leukemia stem cells (BC LSCs) was partially driven by decreased MBNL3. Lentiviral knockdown of MBNL3 resulted in reversion to an embryonic alternative splice isoform program typified by overexpression of CD44 transcript variant 3, containing variant exons 8-10, and BC LSC proliferation. Although isoform-specific lentiviral CD44v3 overexpression enhanced chronic phase chronic myeloid leukemia (CML) progenitor replating capacity, lentiviral shRNA knockdown abrogated these effects. Combined treatment with a humanized pan-CD44 monoclonal antibody and a breakpoint cluster region - ABL proto-oncogene 1, nonreceptor tyrosine kinase (BCR-ABL1) antagonist inhibited LSC maintenance in a niche-dependent manner. In summary, MBNL3 down-regulation-related reversion to an embryonic alternative splicing program, typified by CD44v3 overexpression, represents a previously unidentified mechanism governing malignant progenitor reprogramming in malignant microenvironments and provides a pivotal opportunity for selective BC LSC detection and therapeutic elimination.
Keywords: CD44v3; MBNL3; RNA splicing; adhesion molecules; self-renewal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


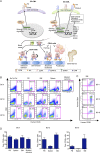


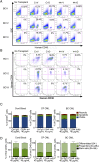
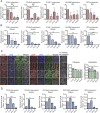
References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. - PubMed
-
- Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. - PubMed
-
- Van Hoof D, et al. Phosphorylation dynamics during early differentiation of human embryonic stem cells. Cell Stem Cell. 2009;5(2):214–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

