Functional screen identifies kinases driving prostate cancer visceral and bone metastasis
- PMID: 26621741
- PMCID: PMC4720329
- DOI: 10.1073/pnas.1521674112
Functional screen identifies kinases driving prostate cancer visceral and bone metastasis
Abstract
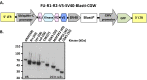
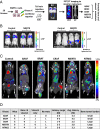
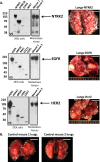
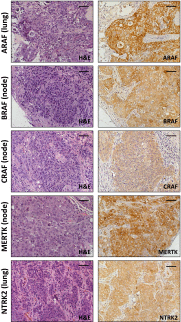
Mutationally activated kinases play an important role in the progression and metastasis of many cancers. Despite numerous oncogenic alterations implicated in metastatic prostate cancer, mutations of kinases are rare. Several lines of evidence suggest that nonmutated kinases and their pathways are involved in prostate cancer progression, but few kinases have been mechanistically linked to metastasis. Using a mass spectrometry-based phosphoproteomics dataset in concert with gene expression analysis, we selected over 100 kinases potentially implicated in human metastatic prostate cancer for functional evaluation. A primary in vivo screen based on overexpression of candidate kinases in murine prostate cells identified 20 wild-type kinases that promote metastasis. We queried these 20 kinases in a secondary in vivo screen using human prostate cells. Strikingly, all three RAF family members, MERTK, and NTRK2 drove the formation of bone and visceral metastasis confirmed by positron-emission tomography combined with computed tomography imaging and histology. Immunohistochemistry of tissue microarrays indicated that these kinases are highly expressed in human metastatic castration-resistant prostate cancer tissues. Our functional studies reveal the strong capability of select wild-type protein kinases to drive critical steps of the metastatic cascade, and implicate these kinases in possible therapeutic intervention.
Keywords: bone metastasis; kinases; metastasis; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Comment in
-
Prostate cancer: A walk on the wild side - wild-type kinases promote metastasis.Nat Rev Urol. 2016 Feb;13(2):63. doi: 10.1038/nrurol.2015.300. Epub 2015 Dec 22. Nat Rev Urol. 2016. PMID: 26689840 No abstract available.
-
Driven to metastasize: Kinases as potential therapeutic targets in prostate cancer.Proc Natl Acad Sci U S A. 2016 Jan 19;113(3):473-5. doi: 10.1073/pnas.1522938113. Epub 2016 Jan 8. Proc Natl Acad Sci U S A. 2016. PMID: 26747602 Free PMC article. No abstract available.
-
Screening for functional kinases in metastatic prostate cancer: a glimmer of hope for kinase inhibition.Transl Androl Urol. 2016 Aug;5(4):616-9. doi: 10.21037/tau.2016.05.13. Transl Androl Urol. 2016. PMID: 27649791 Free PMC article. No abstract available.
References
-
- van Dodewaard-de Jong JM, et al. New treatment options for patients with metastatic prostate cancer: What is the optimal sequence? Clin Genitourin Cancer. 2015;13(4):271–279. - PubMed
-
- Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349(4):366–381. - PubMed
-
- Bubendorf L, et al. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. - PubMed
-
- de Bono JS, et al. TROPIC Investigators Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376(9747):1147–1154. - PubMed
-
- Parker C, et al. ALSYMPCA Investigators Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2P30CA016042-39/CA/NCI NIH HHS/United States
- R01 CA195505/CA/NCI NIH HHS/United States
- R25 CA098010/CA/NCI NIH HHS/United States
- R01 CA181242/CA/NCI NIH HHS/United States
- 1R01CA195505/CA/NCI NIH HHS/United States
- P50 CA097186/CA/NCI NIH HHS/United States
- P50 CA086306/CA/NCI NIH HHS/United States
- P50 CA092131/CA/NCI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- P01 CA085859/CA/NCI NIH HHS/United States
- P50CA97186/CA/NCI NIH HHS/United States
- T32 CA009120/CA/NCI NIH HHS/United States
- P01CA085859/CA/NCI NIH HHS/United States
- Howard Hughes Medical Institute/United States
- T32 CA009056/CA/NCI NIH HHS/United States
- R25T CA098010/CA/NCI NIH HHS/United States
- 1R01CA181242-01A1/CA/NCI NIH HHS/United States
- T32 CA00912/CA/NCI NIH HHS/United States
- R01 CA172603/CA/NCI NIH HHS/United States
- 5R01CA172603-02/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

