Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: a review focusing on mitochondrial respiration and reactive oxygen species
- PMID: 26621844
- PMCID: PMC4868680
- DOI: 10.18632/oncotarget.6405
Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: a review focusing on mitochondrial respiration and reactive oxygen species
Abstract
Although Bcl-2 family proteins were originally identified as key regulators of apoptosis, an impressive body of evidence has shown that pro-survival members of the Bcl-2 family, including Bcl-2, Bcl-XL, and Bcl-w, can also promote cell migration, invasion, and cancer metastasis. Interestingly, cell invasion was recently found to be suppressed by multidomain pro-apoptotic members of the Bcl-2 family, such as Bax and Bak. While the mechanisms underlying these new functions of Bcl-2 proteins are just beginning to be studied, reactive oxygen species (ROS) have emerged as inducers of cell invasion and the production of ROS from mitochondrial respiration is known to be promoted and suppressed by the pro-survival and multidomain pro-apoptotic Bcl-2 family members, respectively. Here, I review the evidence supporting the ability of Bcl-2 proteins to regulate cancer cell invasion and metastasis, and discuss our current understanding of their underlying mechanisms, with a particular focus on mitochondrial respiration and ROS, which could have implications for the development of strategies to overcome tumor progression.
Keywords: Bcl-2 family; cancer invasion and metastasis; mitochondrial respiration; reactive oxygen species.
Conflict of interest statement
The author declares no conflict of interest.
Figures
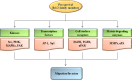


Similar articles
-
Src and epidermal growth factor receptor mediate the pro-invasive activity of Bcl-w.Tumour Biol. 2016 Jan;37(1):1245-52. doi: 10.1007/s13277-015-3917-x. Epub 2015 Aug 20. Tumour Biol. 2016. PMID: 26286833
-
Bcl-w promotes cell invasion by blocking the invasion-suppressing action of Bax.Cell Signal. 2012 Jun;24(6):1163-72. doi: 10.1016/j.cellsig.2012.01.019. Cell Signal. 2012. PMID: 22570867
-
Pro-apoptotic Bax promotes mesenchymal-epithelial transition by binding to respiratory complex-I and antagonizing the malignant actions of pro-survival Bcl-2 proteins.Cancer Lett. 2018 Jun 28;424:127-135. doi: 10.1016/j.canlet.2018.03.033. Epub 2018 Mar 27. Cancer Lett. 2018. PMID: 29596889
-
Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator.Mitochondrion. 2014 Nov;19 Pt A:39-48. doi: 10.1016/j.mito.2014.06.002. Epub 2014 Jun 19. Mitochondrion. 2014. PMID: 24954615 Review.
-
Bcl-2 proteins and mitochondria--specificity in membrane targeting for death.Biochim Biophys Acta. 2011 Apr;1813(4):532-9. doi: 10.1016/j.bbamcr.2010.10.017. Epub 2010 Nov 5. Biochim Biophys Acta. 2011. PMID: 21056595 Review.
Cited by
-
MicroRNA-15a Inhibits Proliferation and Induces Apoptosis in CNE1 Nasopharyngeal Carcinoma Cells.Oncol Res. 2016;24(3):145-51. doi: 10.3727/096504016X14611963142290. Oncol Res. 2016. Retraction in: Oncol Res. 2024 Sep 18;32(10):1683-1684. doi: 10.32604/or.2024.056899. PMID: 27458095 Free PMC article. Retracted.
-
Mitochondrial-Targeted Triphenylphosphonium-Hydroxycamptothecin Conjugate and Its Nano-Formulations for Breast Cancer Therapy: In Vitro and In Vivo Investigation.Pharmaceutics. 2023 Jan 24;15(2):388. doi: 10.3390/pharmaceutics15020388. Pharmaceutics. 2023. PMID: 36839710 Free PMC article.
-
Evaluation of the Cytotoxic Activity and Anti-Migratory Effect of Berberine-Phytantriol Liquid Crystalline Nanoparticle Formulation on Non-Small-Cell Lung Cancer In Vitro.Pharmaceutics. 2022 May 24;14(6):1119. doi: 10.3390/pharmaceutics14061119. Pharmaceutics. 2022. PMID: 35745691 Free PMC article.
-
Synergism Potentiates Oxidative Antiproliferative Effects of Naringenin and Quercetin in MCF-7 Breast Cancer Cells.Nutrients. 2022 Aug 21;14(16):3437. doi: 10.3390/nu14163437. Nutrients. 2022. PMID: 36014942 Free PMC article.
-
In vivo and in vitro induction of the apoptotic effects of oxysophoridine on colorectal cancer cells via the Bcl-2/Bax/caspase-3 signaling pathway.Oncol Lett. 2017 Dec;14(6):8000-8006. doi: 10.3892/ol.2017.7227. Epub 2017 Oct 19. Oncol Lett. 2017. PMID: 29344242 Free PMC article.
References
-
- Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009;21:871–877. - PubMed
-
- Ghiotto F, Fais F, Bruno S. BH3-only proteins: the death-puppeteer's wires. Cytometry A. 2010;77:11–21. - PubMed
-
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. - PubMed
-
- Del Bufalo D, Biroccio A, Leonetti C, Zupi G. Bcl-2 overexpression enhances the metastatic potential of a human breast cancer line. FASEB J. 1997;11:947–953. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

