microRNA-20a enhances the epithelial-to-mesenchymal transition of colorectal cancer cells by modulating matrix metalloproteinases
- PMID: 26622375
- PMCID: PMC4509132
- DOI: 10.3892/etm.2015.2538
microRNA-20a enhances the epithelial-to-mesenchymal transition of colorectal cancer cells by modulating matrix metalloproteinases
Abstract
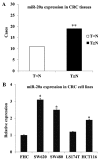
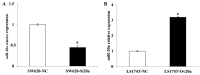
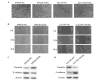
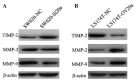
The mortality rates associated with colorectal cancer (CRC) are high due to metastasis. Epithelial-to-mesenchymal transition (EMT) is a key step in tumor metastasis. The aim of the present study was to investigate the function of microRNA-20a (miR-20a) in EMT. The expression of miR-20a was analyzed in CRC tissues and cell lines using the reverse transcription-quantitative polymerase chain reaction. Plasmids containing miR-20a short hairpin RNA and miR-20a mimics were transfected into SW620 and LS174T cell lines, respectively. Cell counting kit-8, Transwell® and wound healing assays were performed to assess the effects of miR-20a on cell proliferation, invasion and migration. EMT markers and matrix metalloproteinases (MMPs) were identified using western blotting. The results showed that increased expression of miR-20a in CRC tissues was associated with tumor invasion and lymph node metastasis (P<0.05). Further experiments indicated that miR-20a-knockdown inhibited the proliferation, invasion and migration of CRC cells, upregulated the expression of vimentin and tissue inhibitor of metalloproteinases-2 (TIMP-2) and downregulated the expression of E-cadherin, MMP-2 and MMP-9. The opposite effects were observed in CRC cell lines overexpressing miR-20a. In conclusion, these results have shown that the upregulation of miR-20a suppresses TIMP-2 expression, which subsequently increases the expression of MMP-2 and MMP-9, thereby promoting the EMT of CRC cells. These findings suggest that miR-20a represents a potential therapeutic target for patients with CRC.
Keywords: colorectal cancer; epithelial-to-mesenchymal transition; microRNA-20a.
Figures

Similar articles
-
MicroRNA-20a-5p promotes colorectal cancer invasion and metastasis by downregulating Smad4.Oncotarget. 2016 Jul 19;7(29):45199-45213. doi: 10.18632/oncotarget.9900. Oncotarget. 2016. PMID: 27286257 Free PMC article.
-
MicroRNA-20a-5p inhibits epithelial to mesenchymal transition and invasion of endometrial cancer cells by targeting STAT3.Int J Clin Exp Pathol. 2018 Dec 1;11(12):5715-5724. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31949657 Free PMC article.
-
Inhibition of miR-20a-5p Suppresses Epithelial-Mesenchymal Transition of Colorectal Cancer Cells Through GJA1.Mol Biotechnol. 2024 Nov 22. doi: 10.1007/s12033-024-01315-2. Online ahead of print. Mol Biotechnol. 2024. PMID: 39576559
-
MicroRNA-128 targeting RPN2 inhibits cell proliferation and migration through the Akt-p53-cyclin pathway in colorectal cancer cells.Oncol Lett. 2018 Dec;16(6):6940-6949. doi: 10.3892/ol.2018.9506. Epub 2018 Sep 26. Oncol Lett. 2018. PMID: 30546426 Free PMC article.
-
Matrix Metalloproteinases in Chemoresistance: Regulatory Roles, Molecular Interactions, and Potential Inhibitors.J Oncol. 2022 May 9;2022:3249766. doi: 10.1155/2022/3249766. eCollection 2022. J Oncol. 2022. PMID: 35586209 Free PMC article. Review.
Cited by
-
The Efficacy of miR-20a as a Diagnostic and Prognostic Biomarker for Colorectal Cancer: A Systematic Review and Meta-Analysis.Cancers (Basel). 2019 Aug 3;11(8):1111. doi: 10.3390/cancers11081111. Cancers (Basel). 2019. PMID: 31382594 Free PMC article. Review.
-
MicroRNA-related transcription factor regulatory networks in human colorectal cancer.Medicine (Baltimore). 2019 Apr;98(15):e15158. doi: 10.1097/MD.0000000000015158. Medicine (Baltimore). 2019. PMID: 30985693 Free PMC article.
-
Post-transcriptional regulation of MMP2 mRNA by its interaction with miR-20a and Nucleolin in breast cancer cell lines.Mol Biol Rep. 2021 Mar;48(3):2315-2324. doi: 10.1007/s11033-021-06261-9. Epub 2021 Mar 31. Mol Biol Rep. 2021. PMID: 33788053
-
Circulating Serum miRNAs as Diagnostic Markers for Colorectal Cancer.PLoS One. 2016 May 2;11(5):e0154130. doi: 10.1371/journal.pone.0154130. eCollection 2016. PLoS One. 2016. PMID: 27135244 Free PMC article.
-
Genetic and molecular origins of colorectal Cancer among the Iranians: an update.Diagn Pathol. 2018 Dec 22;13(1):97. doi: 10.1186/s13000-018-0774-0. Diagn Pathol. 2018. PMID: 30579343 Free PMC article. Review.
References
-
- World Health Organization (WHO), corp-author GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. [Apr 30;2015 ]. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx Accessed.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
