Dimethyl sulfoxide induces chemotherapeutic resistance in the treatment of testicular embryonal carcinomas
- PMID: 26622550
- PMCID: PMC4509453
- DOI: 10.3892/ol.2015.3306
Dimethyl sulfoxide induces chemotherapeutic resistance in the treatment of testicular embryonal carcinomas
Abstract
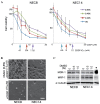
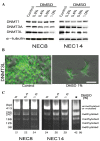
Dimethyl sulfoxide (DMSO) is an amphipathic molecule that is used as a solvent in biological studies and as a vehicle for drug therapy. The present study was designed to evaluate the potential effects of DMSO as a solvent in the treatment of testicular embryonal carcinomas (ECs). DMSO was applied to two human EC cell lines (NEC8 and NEC14), with the treated cells evaluated in relation to cisplatin (CDDP) resistance, differentiation (using Vimentin, Fibronectin, TRA-1-60, and SSEA-1 and -3 as markers) and stemness (denoted by expression of SOX2 and OCT3/4). Furthermore, DNA methyltransferase (DNMT-1, -3A and -3L) expression and methylation status were analyzed. DMSO induced resistance to CDDP, aberrant differentiation and reduction of stemness-related markers in each of the EC cell lines. The expression levels of DNMT-3L and -3A were reduced in response to DMSO, while this treatment also affected DNA methylation. The data demonstrated that DMSO perturbed differentiation, reduced stemness and induced resistance to CDDP in human EC cells. Therefore, DMSO could reduce drug efficacy against EC cells and its use should be carefully managed in the clinical application of chemotherapy.
Keywords: cisplatin; differentiation; dimethyl sulfoxide; embryonal carcinoma; methylation.
Figures

Similar articles
-
Aza-induced cardiomyocyte differentiation of P19 EC-cells by epigenetic co-regulation and ERK signaling.Gene. 2013 Sep 10;526(2):364-73. doi: 10.1016/j.gene.2013.05.044. Epub 2013 Jun 7. Gene. 2013. PMID: 23747406
-
Dimethyl sulfoxide has an impact on epigenetic profile in mouse embryoid body.Stem Cells. 2006 Nov;24(11):2549-56. doi: 10.1634/stemcells.2005-0427. Epub 2006 Jul 13. Stem Cells. 2006. PMID: 16840553
-
Diverse effects of dimethyl sulfoxide (DMSO) on the differentiation potential of human embryonic stem cells.Arch Toxicol. 2012 Apr;86(4):651-61. doi: 10.1007/s00204-011-0782-2. Epub 2011 Nov 22. Arch Toxicol. 2012. PMID: 22105179
-
Reversible G1 arrest induced by dimethyl sulfoxide in human lymphoid cell lines: dimethyl sulfoxide inhibits IL-6-induced differentiation of SKW6-CL4 into IgM-secreting plasma cells.Exp Cell Res. 1996 Jan 10;222(1):218-24. doi: 10.1006/excr.1996.0027. Exp Cell Res. 1996. PMID: 8549666
-
The Rationality of Implementation of Dimethyl Sulfoxide as Differentiation-inducing Agent in Cancer Therapy.Cancer Diagn Progn. 2023 Jan 3;3(1):1-8. doi: 10.21873/cdp.10172. eCollection 2023 Jan-Feb. Cancer Diagn Progn. 2023. PMID: 36632588 Free PMC article. Review.
Cited by
-
Efficacy of EZH2 inhibitory drugs in human papillomavirus-positive and human papillomavirus-negative oropharyngeal squamous cell carcinomas.Clin Epigenetics. 2017 Sep 6;9:95. doi: 10.1186/s13148-017-0390-y. eCollection 2017. Clin Epigenetics. 2017. PMID: 28878842 Free PMC article.
-
Cisplatin Resistance in Testicular Germ Cell Tumors: Current Challenges from Various Perspectives.Cancers (Basel). 2020 Jun 17;12(6):1601. doi: 10.3390/cancers12061601. Cancers (Basel). 2020. PMID: 32560427 Free PMC article. Review.
-
Dimethyl Sulfoxide Perturbs Cell Cycle Progression and Spindle Organization in Porcine Meiotic Oocytes.PLoS One. 2016 Jun 27;11(6):e0158074. doi: 10.1371/journal.pone.0158074. eCollection 2016. PLoS One. 2016. PMID: 27348312 Free PMC article.
-
SEC24A facilitates colocalization and Ca2+ flux between the endoplasmic reticulum and mitochondria.J Cell Sci. 2021 Mar 26;134(6):jcs249276. doi: 10.1242/jcs.249276. J Cell Sci. 2021. PMID: 33622772 Free PMC article.
-
Cryoprotectant-free cryopreservation of mammalian cells by superflash freezing.Proc Natl Acad Sci U S A. 2019 Apr 16;116(16):7738-7743. doi: 10.1073/pnas.1808645116. Epub 2019 Apr 1. Proc Natl Acad Sci U S A. 2019. PMID: 30936320 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
