Effect of adipose-derived stem cell-conditioned medium on the proliferation and migration of B16 melanoma cells
- PMID: 26622561
- PMCID: PMC4508978
- DOI: 10.3892/ol.2015.3360
Effect of adipose-derived stem cell-conditioned medium on the proliferation and migration of B16 melanoma cells
Abstract
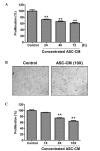
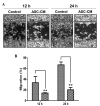
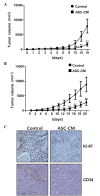
Adipose-derived stem cells (ASCs) are a population of cells derived from adipose tissue. ASCs exhibit multilineage development potential and are able to secrete various factors, which influence adjacent cells. Previous studies have reported the effectiveness of ASC-conditioned medium (ASC-CM) in wound healing, anti-melanogenesis, wrinkle improvement and hair growth. In the present study, the anticancer function of ASC-CM was investigated in vitro and in vivo. An MTT assay revealed that ASC-CM significantly decreased the proliferation of B16 melanoma cells in a time- and dose-dependent manner (P<0.01). Cell cycle analysis indicated that ASC-CM significantly increased the number of cells in G1 phase while reducing the number of cells in the S and G2/M phases (P<0.01). Furthermore, a wound migration model demonstrated that ASC-CM treatment significantly decreased the migration ability of B16 melanoma cells (P<0.01). In addition, C57BL/6 mice were administered with a single intratumoral injection of ASC-CM, daily or every other day, and a significant reduction in the volume of the tumor mass was observed compared with that of the control group (P<0.01). Thus, the findings of the present study indicated that ASC-CM has an anti-tumorigenic effect on B16 melanoma cells in vitro and in vivo, and may potentially be used to support the treatment of melanoma in the future.
Keywords: B16 melanoma cells; adipose-derived stem cell-conditioned media; adipose-derived stem cells; melanoma.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
