Oviductal estrogen receptor α signaling prevents protease-mediated embryo death
- PMID: 26623518
- PMCID: PMC4718728
- DOI: 10.7554/eLife.10453
Oviductal estrogen receptor α signaling prevents protease-mediated embryo death
Abstract
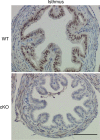
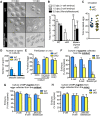
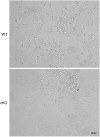
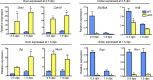
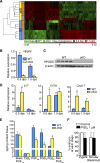
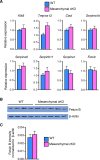
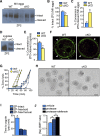
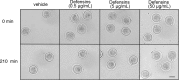
Development of uterine endometrial receptivity for implantation is orchestrated by cyclic steroid hormone-mediated signals. It is unknown if these signals are necessary for oviduct function in supporting fertilization and preimplantation development. Here we show that conditional knockout (cKO) mice lacking estrogen receptor α (ERα) in oviduct and uterine epithelial cells have impaired fertilization due to a dramatic reduction in sperm migration. In addition, all successfully fertilized eggs die before the 2-cell stage due to persistence of secreted innate immune mediators including proteases. Elevated protease activity in cKO oviducts causes premature degradation of the zona pellucida and embryo lysis, and wild-type embryos transferred into cKO oviducts fail to develop normally unless rescued by concomitant transfer of protease inhibitors. Thus, suppression of oviductal protease activity mediated by estrogen-epithelial ERα signaling is required for fertilization and preimplantation embryo development. These findings have implications for human infertility and post-coital contraception.
Keywords: developmental biology; estrogen receptor; fertilization; innate immunity; mouse; oviduct; preimplantation embryo; stem cells.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

