Prohibitin overexpression improves myocardial function in diabetic cardiomyopathy
- PMID: 26623724
- PMCID: PMC4807983
- DOI: 10.18632/oncotarget.6384
Prohibitin overexpression improves myocardial function in diabetic cardiomyopathy
Abstract
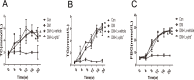
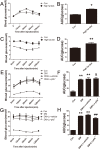
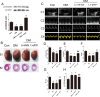
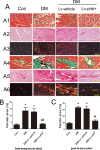
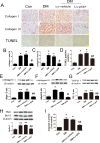
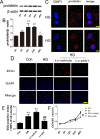
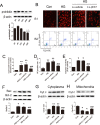
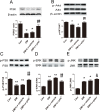
Prohibitin (PHB) is a highly conserved protein implicated in various cellular functions including proliferation, apoptosis, tumor suppression, transcription, and mitochondrial protein folding. However, its function in diabetic cardiomyopathy (DCM) is still unclear. In vivo, type 2 diabetic rat model was induced by using a high-fat diet and low-dose streptozotocin. Overexpression of the PHB protein in the model rats was achieved by injecting lentivirus carrying PHB cDNA via the jugular vein. Characteristics of type 2 DCM were evaluated by metabolic tests, echocardiography and histopathology. Rats with DCM showed severe insulin resistance, left ventricular dysfunction, fibrosis and apoptosis. PHB overexpression ameliorated the disease. Cardiofibroblasts (CFs) and H9c2 cardiomyoblasts were used in vitro to investigate the mechanism of PHB in altered function. In CFs treated with HG, PHB overexpression decreased expression of collagen, matrix metalloproteinase activity, and proliferation. In H9c2 cardiomyoblasts, PHB overexpression inhibited apoptosis induced by HG. Furthermore, the increased phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 was significantly decreased and the inhibited phosphorylation of Akt was restored in DCM. Therefore, PHB may be a new therapeutic target for human DCM.
Keywords: Pathology Section; apoptosis; diabetic cardiomyopathy; myocardial fibrosis; prohibitin.
Conflict of interest statement
None declared.
Figures

References
-
- Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: The search for a unifying hypothesis. Circulation Research. 2006;98:596–605. - PubMed
-
- Schannwell C, Schneppenheim M, Perings S, Plehn G, Strauer B. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology. 2002;588:33–39. - PubMed
-
- Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. Journal of the American College of Cardiology. 2006;47:693–700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

