Enhanced p122RhoGAP/DLC-1 Expression Can Be a Cause of Coronary Spasm
- PMID: 26624289
- PMCID: PMC4666625
- DOI: 10.1371/journal.pone.0143884
Enhanced p122RhoGAP/DLC-1 Expression Can Be a Cause of Coronary Spasm
Abstract
Background: We previously showed that phospholipase C (PLC)-δ1 activity was enhanced by 3-fold in patients with coronary spastic angina (CSA). We also reported that p122Rho GTPase-activating protein/deleted in liver cancer-1 (p122RhoGAP/DLC-1) protein, which was discovered as a PLC-δ1 stimulator, was upregulated in CSA patients. We tested the hypothesis that p122RhoGAP/DLC-1 overexpression causes coronary spasm.
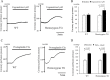
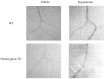
Methods and results: We generated transgenic (TG) mice with vascular smooth muscle (VSM)-specific overexpression of p122RhoGAP/DLC-1. The gene and protein expressions of p122RhoGAP/DLC-1 were markedly increased in the aorta of homozygous TG mice. Stronger staining with anti-p122RhoGAP/DLC-1 in the coronary artery was found in TG than in WT mice. PLC activities in the plasma membrane fraction and the whole cell were enhanced by 1.43 and 2.38 times, respectively, in cultured aortic vascular smooth muscle cells from homozygous TG compared with those from WT mice. Immediately after ergometrine injection, ST-segment elevation was observed in 1 of 7 WT (14%), 6 of 7 heterozygous TG (84%), and 7 of 7 homozygous TG mice (100%) (p<0.05, WT versus TGs). In the isolated Langendorff hearts, coronary perfusion pressure was increased after ergometrine in TG, but not in WT mice, despite of the similar response to prostaglandin F2α between TG and WT mice (n = 5). Focal narrowing of the coronary artery after ergometrine was documented only in TG mice.
Conclusions: VSM-specific overexpression of p122RhoGAP/DLC-1 enhanced coronary vasomotility after ergometrine injection in mice, which is relevant to human CSA.
Conflict of interest statement
Figures




References
-
- Oliva PB, Potts DE, Pluss RG. Coronary arterial spasm in Prinzmetal angina. Documentation by coronary arteriography. N Engl J Med. 1973;288: 745–751. - PubMed
-
- Yasue H, Touyama M, Shimamoto M, Kato H, Tanaka S. Role of autonomic nervous system in the pathogenesis of Prinzmetal's variant form of angina. Circulation. 1974;50: 534–539. - PubMed
-
- Kaku B, Katsuda S, Taguchi T. Life-threatening ventricular arrhythmia due to silent coronary artery spasm: usefulness of I-123 metaiodobenzylguanidine scintigraphy for detecting coronary artery spasm in the era of automated external defibrillators: a case report. J Med Case Rep. BioMed Central Ltd; 2015;9: 26 10.1186/1752-1947-9-26 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

