Novel variants in MLL confer to bladder cancer recurrence identified by whole-exome sequencing
- PMID: 26625313
- PMCID: PMC4823060
- DOI: 10.18632/oncotarget.6380
Novel variants in MLL confer to bladder cancer recurrence identified by whole-exome sequencing
Abstract
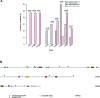
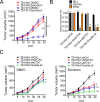
Bladder cancer (BC) is distinguished by high rate of recurrence after surgery, but the underlying mechanisms remain poorly understood. Here we performed the whole-exome sequencing of 37 BC individuals including 20 primary and 17 recurrent samples in which the primary and recurrent samples were not from the same patient. We uncovered that MLL, EP400, PRDM2, ANK3 and CHD5 exclusively altered in recurrent BCs. Specifically, the recurrent BCs and bladder cancer cells with MLL mutation displayed increased histone H3 tri-methyl K4 (H3K4me3) modification in tissue and cell levels and showed enhanced expression of GATA4 and ETS1 downstream. What's more, MLL mutated bladder cancer cells obtained with CRISPR/Cas9 showed increased ability of drug-resistance to epirubicin (a chemotherapy drug for bladder cancer) than wild type cells. Additionally, the BC patients with high expression of GATA4 and ETS1 significantly displayed shorter lifespan than patients with low expression. Our study provided an overview of the genetic basis of recrudescent bladder cancer and discovered that genetic alterations of MLL were involved in BC relapse. The increased modification of H3K4me3 and expression of GATA4 and ETS1 would be the promising targets for the diagnosis and therapy of relapsed bladder cancer.
Keywords: MLL; bladder cancer; drug-resistance; tumor recurrence; whole-exome sequencing.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Chavan S, Bray F, Lortet-Tieulent J, Goodman M, Jemal A. International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66(1):59–73. - PubMed
-
- Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15(1):25–41. - PubMed
-
- Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European urology. 2006;49(3):466–465. discussion 475-467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

