A randomized controlled trial of co-payment elimination: the CHORD trial
- PMID: 26625505
- PMCID: PMC6142179
A randomized controlled trial of co-payment elimination: the CHORD trial
Abstract
Objectives: Efforts to improve adherence by reducing co-payments through value-based insurance design are become more prevalent despite limited evidence of improved health outcomes. The objective of this study was to determine whether eliminating patient co-payments for blood pressure medications improves blood pressure control.
Study design: Randomized controlled trial.
Methods: The Collaboration to Reduce Disparities in Hypertension (CHORD) was a randomized controlled trial with 12 months' follow-up conducted among patients from the Philadelphia and Pittsburgh Veterans Administration Medical Centers. We enrolled 479 patients with poorly controlled systolic blood pressure. Participants were randomly assigned to: a) receive reductions in co-payments from $8 to $0 per medication per month for each antihypertensive prescription filled, b) a computerized behavioral intervention (CBI), c) both co-pay reduction and CBI, or d) usual care. Our main outcome measure was change in systolic blood pressure from enrollment to 12 months post enrollment. We also measured adherence using the medication possession ratio in a subset of participants.
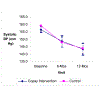
Results: There were no significant interactions between the co-payment interventions and the CBI interventions. There was no relative difference in the change in medication possession ratio between baseline and 12 months (0.05% and -.90% in control and incentive groups, respectively; P = .74) or in continuous medication gaps of 30, 60, or 90 days. Blood pressure decreased among all participants, but to a similar degree between the financial incentive and control groups. Systolic pressure within the incentive group dropped 13.2 mm Hg versus 15.2 mm Hg for the control group (difference = 2.0; 95% CI, -2.3 to 6.3; P = .36). The proportion of patients with blood pressure under control at 12 months was 29.5% in the incentive group versus 33.9 in the control group (odds ratio, 0.8; 95% CI, 0.5-1.3; P = .36).
Conclusions: Among patients with poorly controlled blood pressure, financial incentives--as implemented in this trial--that reduced patient cost sharing for blood pressure medications did not improve medication adherence or blood pressure control.
Trial registration: ClinicalTrials.gov NCT00133068.
Figures
References
-
- The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997;157:2413–46. - PubMed
-
- Malik S, Lopez V, Chen R, Wu W, Wong ND. Undertreatment of cardiovascular risk factors among persons with diabetes in the United States. Diabetes Res Clin Pract 2007;77:126–33. - PubMed
-
- Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med 2006;166:1842–7. - PubMed
-
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. - PubMed
-
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA : the journal of the American Medical Association 2002;288:462–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical