Transformable liquid-metal nanomedicine
- PMID: 26625944
- PMCID: PMC4686762
- DOI: 10.1038/ncomms10066
Transformable liquid-metal nanomedicine
Abstract
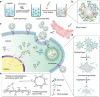
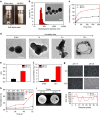
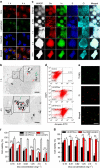
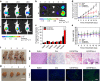
To date, numerous inorganic nanocarriers have been explored for drug delivery systems (DDSs). However, the clinical application of inorganic formulations has often been hindered by their toxicity and failure to biodegrade. We describe here a transformable liquid-metal nanomedicine, based on a core-shell nanosphere composed of a liquid-phase eutectic gallium-indium core and a thiolated polymeric shell. This formulation can be simply produced through a sonication-mediated method with bioconjugation flexibility. The resulting nanoparticles loaded with doxorubicin (Dox) have an average diameter of 107 nm and demonstrate the capability to fuse and subsequently degrade under a mildly acidic condition, which facilitates release of Dox in acidic endosomes after cellular internalization. Equipped with hyaluronic acid, a tumour-targeting ligand, this formulation displays enhanced chemotherapeutic inhibition towards the xenograft tumour-bearing mice. This liquid metal-based DDS with fusible and degradable behaviour under physiological conditions provides a new strategy for engineering theranostic agents with low toxicity.
Figures
References
-
- Huang H.-C., Barua S., Sharma G., Dey S. K. & Rege K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 155, 344–357 (2011). - PubMed
-
- Langille M. R., Personick M. L., Zhang J. & Mirkin C. A. Defining rules for the shape evolution of gold nanoparticles. J. Am. Chem. Soc. 134, 14542–14554 (2012). - PubMed
-
- Sun T. et al. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Engl. 53, 12320–12364 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

