Herpes simplex virus 1 UL41 protein abrogates the antiviral activity of hZAP by degrading its mRNA
- PMID: 26625984
- PMCID: PMC4666169
- DOI: 10.1186/s12985-015-0433-y
Herpes simplex virus 1 UL41 protein abrogates the antiviral activity of hZAP by degrading its mRNA
Abstract
Background: The zinc finger antiviral protein (ZAP) is a host restriction factor that inhibits the replication of various viruses by degradation of certain viral mRNA. However, previous study demonstrated that ectopic expression of rat ZAP did not suppress the replication of herpes simplex virus type 1 (HSV-1), an archetypal member of the alphaherpesvirus subfamily, and the molecular mechanism underneath is still illusive.
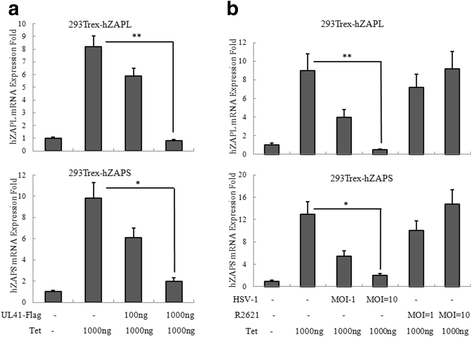
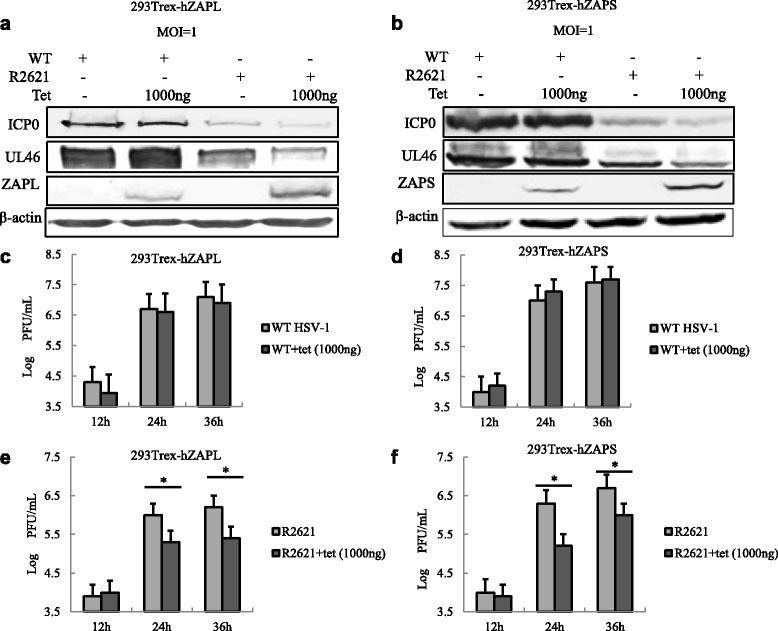
Results: Human ZAP (hZAP) does not suppress the replication of herpes simplex virus 1, and HSV-1 UL41 protein was identified as an antagonist of hZAP by degrading its mRNA. Infection of wild-type (WT), but not UL41-null mutant (R2621) virus, diminished the accumulation of hZAP to abrogate its antiviral activity. Moreover, ectopic expression of hZAP inhibited the replication of R2621 but not WT HSV-1.
Conclusion: HSV-1 UL41 was shown for the first time to evade the antiviral function of hZAP via its RNase activity.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

