M1 and M3 muscarinic receptors may play a role in the neurotoxicity of anhydroecgonine methyl ester, a cocaine pyrolysis product
- PMID: 26626425
- PMCID: PMC4667193
- DOI: 10.1038/srep17555
M1 and M3 muscarinic receptors may play a role in the neurotoxicity of anhydroecgonine methyl ester, a cocaine pyrolysis product
Abstract
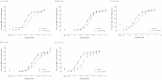
The smoke of crack cocaine contains cocaine and its pyrolysis product, anhydroecgonine methyl ester (AEME). AEME possesses greater neurotoxic potential than cocaine and an additive effect when they are combined. Since atropine prevented AEME-induced neurotoxicity, it has been suggested that its toxic effects may involve the muscarinic cholinergic receptors (mAChRs). Our aim is to understand the interaction between AEME and mAChRs and how it can lead to neuronal death. Using a rat primary hippocampal cell culture, AEME was shown to cause a concentration-dependent increase on both total [(3)H]inositol phosphate and intracellular calcium, and to induce DNA fragmentation after 24 hours of exposure, in line with the activation of caspase-3 previously shown. Additionally, we assessed AEME activity at rat mAChR subtypes 1-5 heterologously expressed in Chinese Hamster Ovary cells. l-[N-methyl-(3)H]scopolamine competition binding showed a preference of AEME for the M2 subtype; calcium mobilization tests revealed partial agonist effects at M1 and M3 and antagonist activity at the remaining subtypes. The selective M1 and M3 antagonists and the phospholipase C inhibitor, were able to prevent AEME-induced neurotoxicity, suggesting that the toxicity is due to the partial agonist effect at M1 and M3 mAChRs, leading to DNA fragmentation and neuronal death by apoptosis.
Figures






References
-
- UNODC (United Nations Office on Drugs and Crime), World Drug Report (2014). United Nations Publication, Sales No., E.14.XI.7. Available at: http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf (Date of access: 04/09/2014).
-
- Schwartz B. G., Rezkalla S. & Kloner R. A. Cardiovascular effects of cocaine. Circulation 122, 2558–2569 (2010). - PubMed
-
- Mao J. T. et al. Cocaine inhibits human endothelial cell IL-8 production: the role of transforming growth factor-β. Cell Immunol 181(1), 38–43 (1997). - PubMed
-
- Valente M. J., Carvalho F., Bastos Md., de Pinho P. G. & Carvalho M. Contribution of oxidative metabolism to cocaine-induced liver and kidney damage. Curr Med Chem 19(33), 5601–5606 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

