Alzheimer therapy with an antibody against N-terminal Abeta 4-X and pyroglutamate Abeta 3-X
- PMID: 26626428
- PMCID: PMC4667289
- DOI: 10.1038/srep17338
Alzheimer therapy with an antibody against N-terminal Abeta 4-X and pyroglutamate Abeta 3-X
Abstract
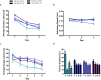
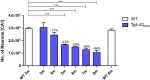
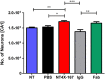
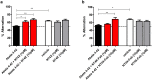
Full-length Aβ1-42 and Aβ1-40, N-truncated pyroglutamate Aβ3-42 and Aβ4-42 are major variants in the Alzheimer brain. Aβ4-42 has not been considered as a therapeutic target yet. We demonstrate that the antibody NT4X and its Fab fragment reacting with both the free N-terminus of Aβ4-x and pyroglutamate Aβ3-X mitigated neuron loss in Tg4-42 mice expressing Aβ4-42 and completely rescued spatial reference memory deficits after passive immunization. NT4X and its Fab fragment also rescued working memory deficits in wild type mice induced by intraventricular injection of Aβ4-42. NT4X reduced pyroglutamate Aβ3-x, Aβx-40 and Thioflavin-S positive plaque load after passive immunization of 5XFAD mice. Aβ1-x and Aβx-42 plaque deposits were unchanged. Importantly, for the first time, we demonstrate that passive immunization using the antibody NT4X is therapeutically beneficial in Alzheimer mouse models showing that N-truncated Aβ starting with position four in addition to pyroglutamate Aβ3-x is a relevant target to fight Alzheimer's disease.
Conflict of interest statement
The antibody NT4X and the animal model Tg4-42 are subject of patent applications.
Figures




References
-
- Selkoe D. J. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol. 8, 447–453 (1998). - PubMed
-
- Selkoe D. J. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–766 (2001). - PubMed
-
- Klein W. L. Abeta toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem. Int. 41, 345–352 (2002). - PubMed
-
- Haass C. & Selkoe D. J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell. Biol. 8, 101–112 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

