Mosquito Defense Strategies against Viral Infection
- PMID: 26626596
- PMCID: PMC4767563
- DOI: 10.1016/j.pt.2015.09.009
Mosquito Defense Strategies against Viral Infection
Abstract
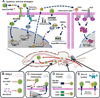
Mosquito-borne viral diseases are a major concern of global health and result in significant economic losses in many countries. As natural vectors, mosquitoes are very permissive to and allow systemic and persistent arbovirus infection. Intriguingly, persistent viral propagation in mosquito tissues neither results in dramatic pathological sequelae nor impairs the vectorial behavior or lifespan, indicating that mosquitoes have evolved mechanisms to tolerate persistent infection and developed efficient antiviral strategies to restrict viral replication to nonpathogenic levels. Here we provide an overview of recent progress in understanding mosquito antiviral immunity and advances in the strategies by which mosquitoes control viral infection in specific tissues.
Keywords: antiviral immunity; arbovirus; mosquito.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

