Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial
- PMID: 26626617
- PMCID: PMC4879109
- DOI: 10.1016/j.eururo.2015.10.049
Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial
Abstract
Background: Sunitinib and everolimus are standard first-line and second-line therapies, respectively, in clear cell renal cell carcinoma (ccRCC).
Objective: To conduct a randomized phase 2 trial comparing sunitinib and everolimus in non-clear cell RCC (non-ccRCC).
Design, setting, and participants: Patients with metastatic, non-ccRCC, or ccRCC with >20% sarcomatoid features (ccSRCC) were randomized to receive sunitinib or everolimus with crossover at disease progression.
Outcome measurement and statistical analysis: Primary end point was progression-free survival (PFS) in first-line therapy; 108 patients were needed to show improvement in median PFS (mPFS) from 12 wk with sunitinib to 20 wk with everolimus.
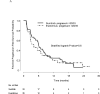
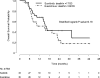
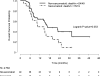
Results and limitations: Interim analysis of 68 patients (papillary [27], chromophobe [12], unclassified [10], translocation [7], ccSRCC [12]) prompted early trial closure. The mPFS in first-line therapy was 6.1 mo with sunitinib and 4.1 mo with everolimus (p=0.6); median overall survival (mOS) was not reached with sunitinib and was 10.5 mo with everolimus, respectively (p=0.014). At final analysis, mOS was 16.2 and 14.9 mo with sunitinib and everolimus, respectively (p=0.18). There were four partial responses (PRs) in first-line therapy (sunitinib: 3 of 33 [9%]; everolimus, 1 of 35 [2.8%]) and four PRs in second-line therapy (sunitinib: 2 of 21 [9.5%]; everolimus, 2 of 23 [8.6%]), with mPFS of 1.8 mo and 2.8 mo, respectively. In patients without sarcomatoid features in their tumors (n=49), mOS was 31.6 mo with sunitinib and 10.5 mo with everolimus (p=0.075). Genomic profiling of a chromophobe RCC from a patient with a PR to first-line everolimus revealed a somatic TSC2 mutation.
Conclusions: In this trial, everolimus was not superior to sunitinib. Both agents demonstrated modest efficacy, underscoring the need for better therapies in non-ccRCC.
Patient summary: This randomized phase 2 trial provides the first head-to-head comparison of everolimus and sunitinib in patients with metastatic non-clear cell renal cell carcinoma (non-ccRCC). The observed very modest efficacy underscores the need to develop more effective therapies for non-ccRCC.
Keywords: Everolimus; Non–clear cell renal cell carcinoma; Renal cell carcinoma; Sunitinib.
Copyright © 2015 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures

Comment in
-
Comparing Everolimus to Sunitinib in Non-clear-cell Renal Cell Carcinoma.Eur Urol. 2016 May;69(5):875-6. doi: 10.1016/j.eururo.2015.11.013. Epub 2015 Nov 26. Eur Urol. 2016. PMID: 26626618 No abstract available.
-
Re: Nizar M. Tannir, Eric Jonasch, Laurence Albiges, et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN):A Randomized Multicenter Phase 2 Trial. Eur Urol 2016;69:866-74.Eur Urol. 2017 Jan;71(1):e23-e24. doi: 10.1016/j.eururo.2016.06.016. Epub 2016 Jun 22. Eur Urol. 2017. PMID: 27344295 No abstract available.
References
-
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–90. - PubMed
-
- Lopez-Beltran A, Carrasco JC, Cheng L, Scarpelli M, Kirkali Z, Montironi R. 2009 update on the classification of renal epithelial tumors in adults. Int J Urol. 2009;16:432–43. - PubMed
-
- Kidney cancer, version 3. National Comprehensive Cancer Network Web site; 2014. [June 23, 2014]. NCCN clinical practice guidelines in oncology (NCCN Guidelines®). http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf.
-
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii49–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

