Lin- CD34hi CD117int/hi FcεRI+ cells in human blood constitute a rare population of mast cell progenitors
- PMID: 26626992
- PMCID: PMC4731844
- DOI: 10.1182/blood-2015-06-650648
Lin- CD34hi CD117int/hi FcεRI+ cells in human blood constitute a rare population of mast cell progenitors
Abstract
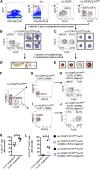
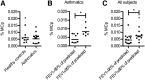
Mast cells are rare tissue-resident immune cells that are involved in allergic reactions, and their numbers are increased in the lungs of asthmatics. Murine lung mast cells arise from committed bone marrow-derived progenitors that enter the blood circulation, migrate through the pulmonary endothelium, and mature in the tissue. In humans, mast cells can be cultured from multipotent CD34(+) progenitor cells. However, a population of distinct precursor cells that give rise to mast cells has remained undiscovered. To our knowledge, this is the first report of human lineage-negative (Lin(-)) CD34(hi) CD117(int/hi) FcεRI(+) progenitor cells, which represented only 0.0053% of the isolated blood cells in healthy individuals. These cells expressed integrin β7 and developed a mast cell-like phenotype, although with a slow cell division capacity in vitro. Isolated Lin(-) CD34(hi) CD117(int/hi) FcεRI(+) blood cells had an immature mast cell-like appearance and expressed high levels of many mast cell-related genes as compared with human blood basophils in whole-transcriptome microarray analyses. Furthermore, serglycin, tryptase, and carboxypeptidase A messenger RNA transcripts were detected by quantitative reverse transcription-polymerase chain reaction. Altogether, we propose that the Lin(-) CD34(hi) CD117(int/hi) FcεRI(+) blood cells are closely related to human tissue mast cells and likely constitute an immediate precursor population, which can give rise to predominantly mast cells. Furthermore, asthmatics with reduced lung function had a higher frequency of Lin(-) CD34(hi) CD117(int/hi) FcεRI(+) blood mast cell progenitors than asthmatics with normal lung function.
© 2016 by The American Society of Hematology.
Figures



Comment in
-
Mast cell differentiation: still open questions?Blood. 2016 Jan 28;127(4):373-4. doi: 10.1182/blood-2015-12-686592. Blood. 2016. PMID: 26823506
References
-
- Khan BQ, Kemp SF. Pathophysiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2011;11(4):319–325. - PubMed
-
- Wenzel SE, Fowler AA, III, Schwartz LB. Activation of pulmonary mast cells by bronchoalveolar allergen challenge. In vivo release of histamine and tryptase in atopic subjects with and without asthma. Am Rev Respir Dis. 1988;137(5):1002–1008. - PubMed
-
- Murray JJ, Tonnel AB, Brash AR, et al. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med. 1986;315(13):800–804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

