The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction
- PMID: 26627720
- PMCID: PMC4720319
- DOI: 10.1073/pnas.1514282112
The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction
Abstract
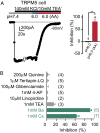
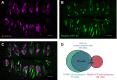
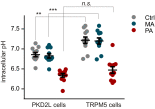
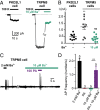
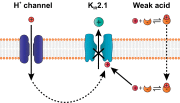
Sour taste is detected by a subset of taste cells on the tongue and palate epithelium that respond to acids with trains of action potentials. Entry of protons through a Zn(2+)-sensitive proton conductance that is specific to sour taste cells has been shown to be the initial event in sour taste transduction. Whether this conductance acts in concert with other channels sensitive to changes in intracellular pH, however, is not known. Here, we show that intracellular acidification generates excitatory responses in sour taste cells, which can be attributed to block of a resting K(+) current. We identify KIR2.1 as the acid-sensitive K(+) channel in sour taste cells using pharmacological and RNA expression profiling and confirm its contribution to sour taste with tissue-specific knockout of the Kcnj2 gene. Surprisingly, acid sensitivity is not conferred on sour taste cells by the specific expression of Kir2.1, but by the relatively small magnitude of the current, which makes the cells exquisitely sensitive to changes in intracellular pH. Consistent with a role of the K(+) current in amplifying the sensory response, entry of protons through the Zn(2+)-sensitive conductance produces a transient block of the KIR2.1 current. The identification in sour taste cells of an acid-sensitive K(+) channel suggests a mechanism for amplification of sour taste and may explain why weak acids that produce intracellular acidification, such as acetic acid, taste more sour than strong acids.
Keywords: gustatory; inward rectifier; potassium channel; proton channel; taste cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
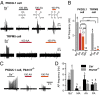
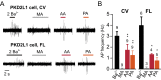
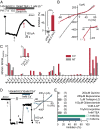
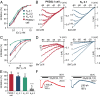
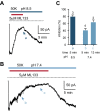

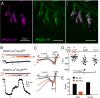
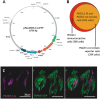
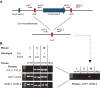



Comment in
-
Sour taste finds closure in a potassium channel.Proc Natl Acad Sci U S A. 2016 Jan 12;113(2):246-7. doi: 10.1073/pnas.1523319113. Epub 2015 Dec 30. Proc Natl Acad Sci U S A. 2016. PMID: 26719421 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 DC004657/DC/NIDCD NIH HHS/United States
- F32 DC013971-01/DC/NIDCD NIH HHS/United States
- R01 HL121706/HL/NHLBI NIH HHS/United States
- P01 HL095488/HL/NHLBI NIH HHS/United States
- T32 HD041697/HD/NICHD NIH HHS/United States
- R21 DC012747/DC/NIDCD NIH HHS/United States
- R01 DC013741/DC/NIDCD NIH HHS/United States
- R01 DC012555/DC/NIDCD NIH HHS/United States
- P30 NS048154/NS/NINDS NIH HHS/United States
- HL121706/HL/NHLBI NIH HHS/United States
- P01 HL-095488/HL/NHLBI NIH HHS/United States
- F32 DC013971/DC/NIDCD NIH HHS/United States
- R37 DK053832/DK/NIDDK NIH HHS/United States
- R37 DK-053832/DK/NIDDK NIH HHS/United States
- R01 HL044455/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

