Non-native Conformers of Cystic Fibrosis Transmembrane Conductance Regulator NBD1 Are Recognized by Hsp27 and Conjugated to SUMO-2 for Degradation
- PMID: 26627832
- PMCID: PMC4722474
- DOI: 10.1074/jbc.M115.685628
Non-native Conformers of Cystic Fibrosis Transmembrane Conductance Regulator NBD1 Are Recognized by Hsp27 and Conjugated to SUMO-2 for Degradation
Abstract
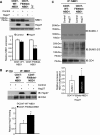
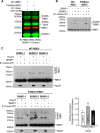
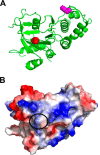
A newly identified pathway for selective degradation of the common mutant of the cystic fibrosis transmembrane conductance regulator (CFTR), F508del, is initiated by binding of the small heat shock protein, Hsp27. Hsp27 collaborates with Ubc9, the E2 enzyme for protein SUMOylation, to selectively degrade F508del CFTR via the SUMO-targeted ubiquitin E3 ligase, RNF4 (RING finger protein 4) (1). Here, we ask what properties of CFTR are sensed by the Hsp27-Ubc9 pathway by examining the ability of NBD1 (locus of the F508del mutation) to mimic the disposal of full-length (FL) CFTR. Similar to FL CFTR, F508del NBD1 expression was reduced 50-60% by Hsp27; it interacted preferentially with the mutant and was modified primarily by SUMO-2. Mutation of the consensus SUMOylation site, Lys(447), obviated Hsp27-mediated F508del NBD1 SUMOylation and degradation. As for FL CFTR and NBD1 in vivo, SUMO modification using purified components in vitro was greater for F508del NBD1 versus WT and for the SUMO-2 paralog. Several findings indicated that Hsp27-Ubc9 targets the SUMOylation of a transitional, non-native conformation of F508del NBD1: (a) its modification decreased as [ATP] increased, reflecting stabilization of the nucleotide-binding domain by ligand binding; (b) a temperature-induced increase in intrinsic fluorescence, which reflects formation of a transitional NBD1 conformation, was followed by its SUMO modification; and (c) introduction of solubilizing or revertant mutations to stabilize F508del NBD1 reduced its SUMO modification. These findings indicate that the Hsp27-Ubc9 pathway recognizes a non-native conformation of mutant NBD1, which leads to its SUMO-2 conjugation and degradation by the ubiquitin-proteasome system.
Keywords: ABC transporter; Prot; SUMO; SUMOylation; chloride channel; cystic fibrosis; cystic fibrosis transmembrane conductance regulator (CFTR); post-translational modification (PTM); protein conformation; protein degradation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Ahner A., Gong X., Schmidt B. Z., Peters K. W., Rabeh W. M., Thibodeau P. H., Lukacs G. L., and Frizzell R. A. (2013) Small heat shock proteins target mutant cystic fibrosis transmembrane conductance regulator for degradation via a small ubiquitin-like modifier-dependent pathway. Mol. Biol. Cell 24, 74–84 - PMC - PubMed
-
- Clancy J. P., Rowe S. M., Accurso F. J., Aitken M. L., Amin R. S., Ashlock M. A., Ballmann M., Boyle M. P., Bronsveld I., Campbell P. W., De Boeck K., Donaldson S. H., Dorkin H. L., Dunitz J. M., Durie P. R., Jain M., Leonard A., McCoy K. S., Moss R. B., Pilewski J. M., Rosenbluth D. B., Rubenstein R. C., Schechter M. S., Botfield M., Ordoñez C. L., Spencer-Green G. T., Vernillet L., Wisseh S., Yen K., and Konstan M. W. (2012) Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 67, 12–18 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

