ATM Localization and Heterochromatin Repair Depend on Direct Interaction of the 53BP1-BRCT2 Domain with γH2AX
- PMID: 26628370
- PMCID: PMC4688034
- DOI: 10.1016/j.celrep.2015.10.074
ATM Localization and Heterochromatin Repair Depend on Direct Interaction of the 53BP1-BRCT2 Domain with γH2AX
Abstract
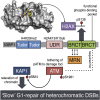
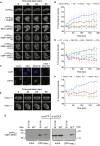
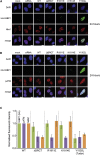
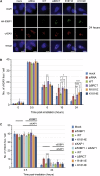
53BP1 plays multiple roles in mammalian DNA damage repair, mediating pathway choice and facilitating DNA double-strand break repair in heterochromatin. Although it possesses a C-terminal BRCT2 domain, commonly involved in phospho-peptide binding in other proteins, initial recruitment of 53BP1 to sites of DNA damage depends on interaction with histone post-translational modifications--H4K20me2 and H2AK13/K15ub--downstream of the early γH2AX phosphorylation mark of DNA damage. We now show that, contrary to current models, the 53BP1-BRCT2 domain binds γH2AX directly, providing a third post-translational mark regulating 53BP1 function. We find that the interaction of 53BP1 with γH2AX is required for sustaining the 53BP1-dependent focal concentration of activated ATM that facilitates repair of DNA double-strand breaks in heterochromatin in G1.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Acs K., Luijsterburg M.S., Ackermann L., Salomons F.A., Hoppe T., Dantuma N.P. The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat. Struct. Mol. Biol. 2011;18:1345–1350. - PubMed
-
- Bakkenist C.J., Kastan M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. - PubMed
-
- Bekker-Jensen S., Mailand N. Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair (Amst.) 2010;9:1219–1228. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

