Identification of Different Classes of Luminal Progenitor Cells within Prostate Tumors
- PMID: 26628377
- PMCID: PMC4840850
- DOI: 10.1016/j.celrep.2015.10.077
Identification of Different Classes of Luminal Progenitor Cells within Prostate Tumors
Abstract
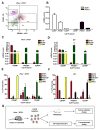
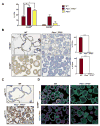
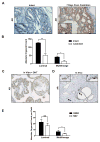
Primary prostate cancer almost always has a luminal phenotype. However, little is known about the stem/progenitor properties of transformed cells within tumors. Using the aggressive Pten/Tp53-null mouse model of prostate cancer, we show that two classes of luminal progenitors exist within a tumor. Not only did tumors contain previously described multipotent progenitors, but also a major population of committed luminal progenitors. Luminal cells, sorted directly from tumors or grown as organoids, initiated tumors of adenocarcinoma or multilineage histological phenotypes, which is consistent with luminal and multipotent differentiation potentials, respectively. Moreover, using organoids we show that the ability of luminal-committed progenitors to self-renew is a tumor-specific property, absent in benign luminal cells. Finally, a significant fraction of luminal progenitors survived in vivo castration. In all, these data reveal two luminal tumor populations with different stem/progenitor cell capacities, providing insight into prostate cancer cells that initiate tumors and can influence treatment response.
Keywords: castration; heterogeneity; luminal; prostate cancer; stem/progenitor cells.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Illuminating the Properties of Prostate Luminal Progenitors.Cell Stem Cell. 2015 Dec 3;17(6):644-646. doi: 10.1016/j.stem.2015.11.018. Cell Stem Cell. 2015. PMID: 26637940
References
-
- Bonizzi G, Cicalese A, Insinga A, Pelicci PG. The emerging role of p53 in stem cells. Trends Mol Med. 2011;18:6–12. - PubMed
-
- Boutros PC, Fraser M, Harding NJ, de Borja R, Trudel D, Lalonde E, Meng A, Hennings-Yeomans PH, McPherson A, Sabelnykova VY, et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat Genet. 2015;47:736–745. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

