Pain exposure physical therapy (PEPT) compared to conventional treatment in complex regional pain syndrome type 1: a randomised controlled trial
- PMID: 26628523
- PMCID: PMC4679993
- DOI: 10.1136/bmjopen-2015-008283
Pain exposure physical therapy (PEPT) compared to conventional treatment in complex regional pain syndrome type 1: a randomised controlled trial
Abstract
Objective: To compare the effectiveness of pain exposure physical therapy (PEPT) with conventional treatment in patients with complex regional pain syndrome type 1 (CRPS-1) in a randomised controlled trial with a blinded assessor.
Setting: The study was conducted at a level 1 trauma centre in the Netherlands.
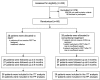
Participants: 56 adult patients with CRPS-1 participated. Three patients were lost to follow-up.
Interventions: Patients received either PEPT in a maximum of five treatment sessions, or conventional treatment following the Dutch multidisciplinary guideline.
Measurements: Outcomes were assessed at baseline and at 3, 6 and 9 months after randomisation. The primary outcome measure was the Impairment level Sum Score--Restricted Version (ISS-RV), consisting of visual analogue scale for pain (VAS-pain), McGill Pain Questionnaire, active range of motion (AROM) and skin temperature. Secondary outcome measures included Pain Disability Index (PDI); muscle strength; Short Form 36 (SF-36); disability of arm, shoulder and hand; Lower Limb Tasks Questionnaire (LLTQ); 10 m walk test; timed up-and-go test (TUG) and EuroQol-5D.
Results: The intention-to-treat analysis showed a clinically relevant decrease in ISS-RV (6.7 points for PEPT and 6.2 points for conventional treatment), but the between-group difference was not significant (0.96, 95% CI -1.56 to 3.48). Participants allocated to PEPT experienced a greater improvement in AROM (between-group difference 0.51, 95% CI 0.07 to 0.94; p=0.02). The per protocol analysis showed larger and significant between-group effects on ISS-RV, VAS-pain, AROM, PDI, SF-36, LLTQ and TUG.
Conclusions: We cannot conclude that PEPT is superior to conventional treatment for patients with CRPS-1. Further high-quality research on the effects of PEPT is warranted given the potential effects as indicated by the per protocol analysis.
Trial registration numbers: NCT00817128 and NTR 2090.
Keywords: PAIN MANAGEMENT; REHABILITATION MEDICINE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

Comment in
-
Durch Schmerz zur Heilung?Z Orthop Unfall. 2016 Feb;154(1):16. Z Orthop Unfall. 2016. PMID: 27340710 German. No abstract available.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
