The Impact of Enhanced Screening and Treatment on Hepatitis C in the United States
- PMID: 26628566
- PMCID: PMC4706637
- DOI: 10.1093/cid/civ894
The Impact of Enhanced Screening and Treatment on Hepatitis C in the United States
Abstract
Background: The effectiveness of interferon-free direct-acting antivirals (DAA) in treating chronic hepatitis C virus (HCV) is limited by low screening and treatment rates, particularly among people who inject drugs (PWIDs).
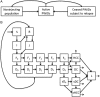
Methods: To evaluate the levels of screening and treatment with interferon-free DAAs that are required to control HCV incidence and HCV-associated morbidity and mortality, we developed a transmission model, stratified by age and by injection drug use, and calibrated it to epidemiological data in the United States from 1992 to 2014. We quantified the impact of administration of DAAs at current and at enhanced screening and treatment rates, focusing on outcomes of HCV incidence, prevalence, compensated and decompensated cirrhosis, hepatocellular carcinoma, liver transplants, and mortality from 2015 to 2040.
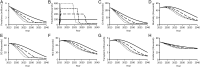
Results: Increasing annual treatment of patients 4-fold-from the approximately 100 000 treated historically to 400 000-is predicted to prevent 526 084 (95% confidence interval, 466 615-593 347) cases of cirrhosis and 256 315 (201 589-316 114) HCV-associated deaths. By simultaneously increasing treatment capacity and increasing the number of HCV infections diagnosed, total HCV prevalence could fall to as low as 305 599 (222 955-422 110) infections by 2040. Complete elimination of HCV transmission in the United States through treatment with DAAs would require nearly universal screening of PWIDs, with an annual treatment rate of at least 30%.
Conclusions: Interferon-free DAAs are projected to achieve marked reductions in HCV-associated morbidity and mortality. Aggressive expansion in HCV screening and treatment, particularly among PWIDs, would be required to eliminate HCV in the United States.
Keywords: direct-acting antivirals; hepatitis C; people who inject drugs; screening; treatment.
© The Author 2015. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures

References
-
- Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int 2011; 31:1090–101. - PubMed
-
- Volk ML, Tocco R, Saini S, Lok ASF. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology 2009; 50:1750–5. - PubMed
-
- Rice CM, Saeed M. Hepatitis C: treatment triumphs. Nature 2014; 510:43–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

