TUSC5 regulates insulin-mediated adipose tissue glucose uptake by modulation of GLUT4 recycling
- PMID: 26629404
- PMCID: PMC4632119
- DOI: 10.1016/j.molmet.2015.08.003
TUSC5 regulates insulin-mediated adipose tissue glucose uptake by modulation of GLUT4 recycling
Abstract
Objective: Failure to properly dispose of glucose in response to insulin is a serious health problem, occurring during obesity and is associated with type 2 diabetes development. Insulin-stimulated glucose uptake is facilitated by the translocation and plasma membrane fusion of vesicles containing glucose transporter 4 (GLUT4), the rate-limiting step of post-prandial glucose disposal.
Methods: We analyzed the role of Tusc5 in the regulation of insulin-stimulated Glut4-mediated glucose uptake in vitro and in vivo. Furthermore, we measured Tusc5 expression in two patient cohorts.
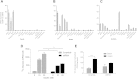
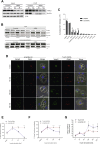
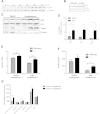
Results: Herein, we report that TUSC5 controls insulin-stimulated glucose uptake in adipocytes, in vitro and in vivo. TUSC5 facilitates the proper recycling of GLUT4 and other key trafficking proteins during prolonged insulin stimulation, thereby enabling proper protein localization and complete vesicle formation, processes that ultimately enable insulin-stimulated glucose uptake. Tusc5 knockout mice exhibit impaired glucose disposal and TUSC5 expression is predictive of glucose tolerance in obese individuals, independent of body weight. Furthermore, we show that TUSC5 is a PPARγ target and in its absence the anti-diabetic effects of TZDs are significantly blunted.
Conclusions: Collectively, these findings establish TUSC5 as an adipose tissue-specific protein that enables proper protein recycling, linking the ubiquitous vesicle traffic machinery with tissue-specific insulin-mediated glucose uptake into adipose tissue and the maintenance of a healthy metabolic phenotype in mice and humans.
Keywords: Glucose uptake; Insulin resistance; Obesity; Type 2 diabetes.
Figures




References
-
- Huang S., Czech M.P. The GLUT4 glucose transporter. Cell Metabolism. 2007;5:237–252. - PubMed
-
- Maier V.H., Gould G.W. Long-term insulin treatment of 3T3-L1 adipocytes results in mis-targeting of GLUT4: implications for insulin-stimulated glucose transport. Diabetologia. 2000;43:1273–1281. - PubMed
-
- Zisman A., Peroni O., Abel E., Michael M., Mauvais-Jarvis F., Lowell B. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nature Medicine. 2000;6:924–928. - PubMed
-
- Abel E.D., Peroni O., Kim J.K., Kim Y.B., Boss O., Hadro E. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

