Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion
- PMID: 26629407
- PMCID: PMC4632112
- DOI: 10.1016/j.molmet.2015.07.009
Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion
Abstract
Objective: Circadian clocks are functional in all light-sensitive organisms, allowing an adaptation to the external world in anticipation of daily environmental changes. In view of the potential role of the skeletal muscle clock in the regulation of glucose metabolism, we aimed to characterize circadian rhythms in primary human skeletal myotubes and investigate their roles in myokine secretion.
Methods: We established a system for long-term bioluminescence recording in differentiated human myotubes, employing lentivector gene delivery of the Bmal1-luciferase and Per2-luciferase core clock reporters. Furthermore, we disrupted the circadian clock in skeletal muscle cells by transfecting siRNA targeting CLOCK. Next, we assessed the basal secretion of a large panel of myokines in a circadian manner in the presence or absence of a functional clock.
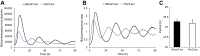
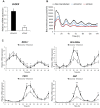
Results: Bioluminescence reporter assays revealed that human skeletal myotubes, synchronized in vitro, exhibit a self-sustained circadian rhythm, which was further confirmed by endogenous core clock transcript expression. Moreover, we demonstrate that the basal secretion of IL-6, IL-8 and MCP-1 by synchronized skeletal myotubes has a circadian profile. Importantly, the secretion of IL-6 and several additional myokines was strongly downregulated upon siClock-mediated clock disruption.
Conclusions: Our study provides for the first time evidence that primary human skeletal myotubes possess a high-amplitude cell-autonomous circadian clock, which could be attenuated. Furthermore, this oscillator plays an important role in the regulation of basal myokine secretion by skeletal myotubes.
Keywords: Circadian bioluminescence recording; Circadian clock; Human skeletal myotube; Interleukin-6; Myokine.
Figures


References
-
- Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–260. - PubMed
-
- Bass J. Circadian topology of metabolism. Nature. 2012;491:348–356. - PubMed
-
- Dibner C., Schibler U. Circadian timing of metabolism in animal models and humans. Journal of Internal Medicine. 2015 May;277(5):513–527. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

