Sex-dependent changes in metabolism and behavior, as well as reduced anxiety after eliminating ventromedial hypothalamus excitatory output
- PMID: 26629409
- PMCID: PMC4632173
- DOI: 10.1016/j.molmet.2015.09.001
Sex-dependent changes in metabolism and behavior, as well as reduced anxiety after eliminating ventromedial hypothalamus excitatory output
Abstract
Objectives: The ventromedial hypothalamic nucleus (VMH) regulates energy homeostasis as well as social and emotional behaviors. Nearly all VMH neurons, including those in the sexually dimorphic ventrolateral VMH (VMHvl) subregion, release the excitatory neurotransmitter glutamate and use the vesicular glutamate transporter 2 (Vglut2). Here, we asked how glutamatergic signaling contributes to the collective metabolic and behavioral responses attributed to the VMH and VMHvl.
Methods: Using Sf1-Cre and a Vglut2 floxed allele, Vglut2 was knocked-out in SF-1 VMH neurons (Vglut2 (Sf1-Cre) ). Metabolic and neurobehavioral assays were carried out initially on Vglut2 (fl/fl) and Vglut2 (Sf1-Cre) mice in a mixed, and then in the C57BL/6 genetic background, which is prone to hyperglycemia and diet induced obesity (DIO).
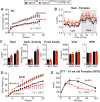
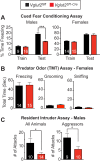
Results: Several phenotypes observed in Vglut2 (Sf1-Cre) mice were largely unexpected based on prior studies that have perturbed VMH development or VMH glutamate signaling. In our hands, Vglut2 (Sf1-Cre) mice failed to exhibit the anticipated increase in body weight after high fat diet (HFD) or the impaired glucose homeostasis after fasting. Instead, there was a significant sex-dependent attenuation of DIO in Vglut2 (Sf1-Cre) females. Vglut2 (Sf1-Cre) males also display a sex-specific loss of conditioned-fear responses and aggression accompanied by more novelty-associated locomotion. Finally, unlike the higher anxiety noted in Sf1 (Nestin-Cre) mice that lack a fully formed VMH, both male and female Vglut2 (Sf1-Cre) mice were less anxious.
Conclusions: Loss of VMH glutamatergic signaling sharply decreased DIO in females, attenuated aggression and learned fear in males, and was anxiolytic in males and females. Collectively, our findings demonstrate that while glutamatergic output from the VMH appears largely dispensable for counter regulatory responses to hypoglycemia, it drives sex-dependent differences in metabolism and social behaviors and is essential for adaptive responses to anxiety-provoking stimuli in both sexes.
Keywords: Anxiety; Excitatory output; Male aggression; Sex-dependent obesity; VGlut2; VMH.
Figures




References
-
- Ziegler D.R., Cullinan W.E., Herman J.P. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. Journal of Comparative Neurology. 2002;448(3):217–229. - PubMed
-
- Fremeau R.T., Jr., Troyer M.D., Pahner I., Nygaard G.O., Tran C.H., Reimer R.J. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31(2):247–260. - PubMed
-
- Sternson S.M., Shepherd G.M., Friedman J.M. Topographic mapping of VMH--> arcuate nucleus microcircuits and their reorganization by fasting. Nature Neuroscience. 2005;8(10):1356–1363. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

