Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities
- PMID: 26629425
- PMCID: PMC4630514
- DOI: 10.3978/j.issn.2218-6751.2015.06.06
Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities
Abstract
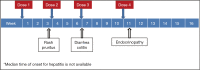
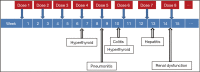
Immune checkpoint blockade using inhibitors of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death-1 (PD-1) has shown clinically significant antitumor response and has been approved for the treatment of malignant melanoma and squamous non-small cell lung cancer (NSCLC). These immunotherapies are associated with unique set of toxicities termed immune-related adverse events (irAEs) that are very different from toxicities observed with conventional cytotoxic chemotherapy. Prompt recognition and initiation of appropriate management, usually in the form of immunosuppression, usually results in complete reversibility, but failing to do so can lead to severe toxicity or even death. Clinical algorithms describing the management of common irAEs have been published based on clinical trial information and experience in metastatic melanoma with ipilimumab, a human IgG1 monoclonal antibody that binds to CTLA-4 and blocks T cell inhibition. The most common irAEs reported with ipilimumab are dermatologic toxicity, diarrhea/colitis, hepatotoxicity, and endocrinopathies, although other sites can also be affected. Similar irAEs have been observed with agents targeting PD-1. Nivolumab and pembrolizumab are humanized monoclonal antibodies that bind to PD-1 and prevent T cell inactivation. Ipilimumab, pembrolizumab, and nivolumab are approved by the Food and Drug Administration (FDA) for the treatment of advanced melanoma; nivolumab was also recently approved for metastatic squamous NSCLC. This review describes the optimal management of toxicities related to immune checkpoint inhibition from FDA-approved agents targeting CTLA-4 and PD-1.
Keywords: Immunotherapy; checkpoint inhibition; toxicity.
Conflict of interest statement
Figures
References
-
- Yervoy® [package insert]. Princeton, NJ, USA: Bristol-Myers Squibb Company, 2013. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2265ef30-253e-11..., accessed June 2015.
-
- Keytruda® [package insert]. Whitehouse Station, NJ, USA: Merck & Co., Inc., 2015. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9333c79b-d487-45..., accessed June 2015.
-
- Opdivo® [package insert]. Princeton, NJ, USA: Bristol-Myers Squibb Company, 2015. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f570b9c4-6846-4d..., accessed June 2015.
-
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical