Long-term response to first-line trabectedin in an elderly female patient with a metastatic leiomyosarcoma unfit for anthracycline
- PMID: 26629769
- PMCID: PMC4736294
- DOI: 10.1097/CAD.0000000000000326
Long-term response to first-line trabectedin in an elderly female patient with a metastatic leiomyosarcoma unfit for anthracycline
Abstract
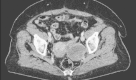
Systemic chemotherapy comprising anthracycline monotherapy is the standard regimen for metastatic soft tissue sarcomas, particularly leiomyosarcomas, which have limited sensitivity to ifosfamide. However, the optimal chemotherapy regimen for elderly patients, especially those considered unfit for anthracyclines, is undefined. Trabectedin is a potent marine-derived antineoplastic drug with documented activity in liposarcomas and leiomyosarcomas. It is registered in Europe for the treatment of adult patients with advanced soft tissue sarcoma, after failure of anthracyclines and ifosfamide, or who are unsuited to receive these agents. We report the long-term response to first-line trabectedin therapy in an elderly patient with metastatic leiomyosarcoma unfit for standard therapy. A 66-year-old woman underwent resection of a pelvic epithelioid leiomyosarcoma with positive margins in December 2002, followed by postoperative radiotherapy. In February 2012, she was diagnosed with multiple lung lesions and local relapse in the pelvis. As she was considered unsuitable for both anthracycline and ifosfamide because of cardiovascular comorbidities and because she was highly anxious at the prospect of developing alopecia, vomiting, and fatigue, we commenced treatment with trabectedin at 75% of the standard dose of 1.5 mg/m every 3 weeks. Treatment was well tolerated, and the patient continued treatment for 25 cycles, with disease stabilization according to the RECIST criteria and a partial response according to the Choi criteria. Disease progression was observed in November 2013 and the patient died 20 months after the diagnosis of metastases. Trabectedin may represent an alternative option for highly selected elderly patients with metastatic sarcoma and unfit for anthracyclines; careful monitoring of toxicities is strongly recommended.
Figures
References
-
- Van Glabbeke M, van Oosterom AT, Oosterhuis JW, Mouridsen H, Crowther D, Somers R, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2185 patients treated with anthracycline-containing first-line regimens – a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 1999; 17:150–157. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2015. CA Cancer J Clin 2015; 65:5–29. - PubMed
-
- Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, et al. European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014; 15:415–423. - PubMed
-
- Von Hoff DD, Layard MW, Basa P, Davis HL, Jr, Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979; 91:710–717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials