Evaluating Models of Cellulose Degradation by Fibrobacter succinogenes S85
- PMID: 26629814
- PMCID: PMC4668043
- DOI: 10.1371/journal.pone.0143809
Evaluating Models of Cellulose Degradation by Fibrobacter succinogenes S85
Abstract
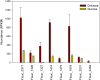
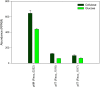
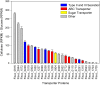
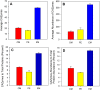
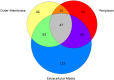
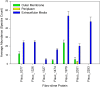
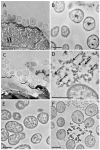
Fibrobacter succinogenes S85 is an anaerobic non-cellulosome utilizing cellulolytic bacterium originally isolated from the cow rumen microbial community. Efforts to elucidate its cellulolytic machinery have resulted in the proposal of numerous models which involve cell-surface attachment via a combination of cellulose-binding fibro-slime proteins and pili, the production of cellulolytic vesicles, and the entry of cellulose fibers into the periplasmic space. Here, we used a combination of RNA-sequencing, proteomics, and transmission electron microscopy (TEM) to further clarify the cellulolytic mechanism of F. succinogenes. Our RNA-sequence analysis shows that genes encoding type II and III secretion systems, fibro-slime proteins, and pili are differentially expressed on cellulose, relative to glucose. A subcellular fractionation of cells grown on cellulose revealed that carbohydrate active enzymes associated with cellulose deconstruction and fibro-slime proteins were greater in the extracellular medium, as compared to the periplasm and outer membrane fractions. TEMs of samples harvested at mid-exponential and stationary phases of growth on cellulose and glucose showed the presence of grooves in the cellulose between the bacterial cells and substrate, suggesting enzymes work extracellularly for cellulose degradation. Membrane vesicles were only observed in stationary phase cultures grown on cellulose. These results provide evidence that F. succinogenes attaches to cellulose fibers using fibro-slime and pili, produces cellulases, such as endoglucanases, that are secreted extracellularly using type II and III secretion systems, and degrades the cellulose into cellodextrins that are then imported back into the periplasm for further digestion by β-glucanases and other cellulases.
Conflict of interest statement
Figures
References
-
- Groleau D, Forsberg CW. Cellulolytic Activity of the Rumen Bacterium Bacteroides-Succinogenes. Can J Microbiol. 1981;27(5):517–30. WOS:A1981LP27900007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

