Levan Enhances Associated Growth of Bacteroides, Escherichia, Streptococcus and Faecalibacterium in Fecal Microbiota
- PMID: 26629816
- PMCID: PMC4667893
- DOI: 10.1371/journal.pone.0144042
Levan Enhances Associated Growth of Bacteroides, Escherichia, Streptococcus and Faecalibacterium in Fecal Microbiota
Abstract
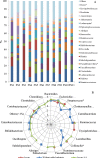
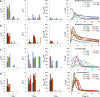
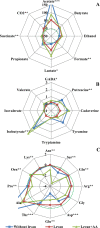
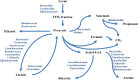
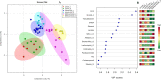
The role of dietary fiber in supporting healthy gut microbiota and overall well-being of the host has been revealed in several studies. Here, we show the effect of a bacterial polyfructan levan on the growth dynamics and metabolism of fecal microbiota in vitro by using isothermal microcalorimetry. Eleven fecal samples from healthy donors were incubated in phosphate-buffered defined medium with or without levan supplementation and varying presence of amino acids. The generation of heat, changes in pH and microbiota composition, concentrations of produced and consumed metabolites during the growth were determined. The composition of fecal microbiota and profile of metabolites changed in response to substrate (levan and amino acids) availability. The main products of levan metabolism were acetic, lactic, butyric, propionic and succinic acids and carbon dioxide. Associated growth of levan-degrading (e.g. Bacteroides) and butyric acid-producing (e.g. Faecalibacterium) taxa was observed in levan-supplemented media. The study shows that the capacity of levan and possibly also other dietary fibers/prebiotics to modulate the composition and function of colon microbiota can be predicted by using isothermal microcalorimetry of fecal samples linked to metabolite and consortia analyses.
Conflict of interest statement
Figures
References
-
- Macfarlane GT, Macfarlane S. Fermentation in the Human Large Intestine. J Clin Gastroenterol. 2011;45(December): S120–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

