Efficacy and Safety of Capecitabine and Oxaliplatin (CapOX) as an Adjuvant Therapy in Japanese for Stage II/III Colon Cancer in a Group at High Risk of Recurrence in Retrospective Study
- PMID: 26629945
- PMCID: PMC7842523
- DOI: 10.3727/096504015X14410238486522
Efficacy and Safety of Capecitabine and Oxaliplatin (CapOX) as an Adjuvant Therapy in Japanese for Stage II/III Colon Cancer in a Group at High Risk of Recurrence in Retrospective Study
Abstract
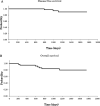
A number of large-scale clinical trials have demonstrated that using a combination of oxaliplatin and fluoropyrimidines as an adjuvant chemotherapy for stage II/III colon cancer improved the prognosis. However, there has only been experience in Japanese patients with using CapOX therapy, in which capecitabine and oxaliplatin are used in combination. Therefore, our objective was to evaluate the efficacy and safety of CapOX in Japanese patients as an adjuvant chemotherapy for colon cancer in a single institute retrospective study. The efficacy and safety of CapOX as an adjuvant chemotherapy for patients with stage III colon cancer and stage II patients who had a signature for high risk of recurrence were evaluated in patients who had undergone surgery at our institution between December 1, 2009 and March 31, 2013. Forty-one patients received CapOX therapy during the study period: 23 men and 18 women with median age of 68.0 years (35-79 years). Performance status was 0 for 33 patients, and PS 1 for eight patients. The clinical stages were stage II in 14 patients, stage IIIA in 15 patients, and stage IIIB in 12 patients. The median number of CapOX cycles was eight (two to eight courses). The treatment completion rate was 82.9%. Five-year DFS rates were 63.8%. Five-year OS rates were 71.0%. In terms of adverse events, the serious adverse events of grade 3 or higher seen among all patients were neutropenia in four patients, thrombocytopenia in one patient, and peripheral sensory neuropathy in seven patients. However, hand-foot syndrome, which is characteristic of capecitabine, was not observed. Efficacy and tolerability of CapOX in Japanese patients as an adjuvant chemotherapy after colon cancer surgery was demonstrated.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Wolmark N.; Rockette H.; Mamounas E.; Jones J.; Wieand S.; Wickerham D. L.; Bear H. D.; Atkins J. N.; Dimitrov N. V.; Glass A.; Fisher E. R.; Fisher B. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project C-04. J. Clin. Oncol. 17:3553–3559; 1999. - PubMed
-
- Saltz L. B.; Niedzwiecki D.; Hollis D.; Goldberg R. M.; Hantel A.; Thomas J. P.; Fields A. L.; Mayer R. J. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J. Clin. Oncol. 25:3456–3461; 2007. - PubMed
-
- Van Cutsem E.; Labianca R.; Bodoky G.; Barone C.; Aranda E.; Nordlinger B.; Topham C.; Tabernero J.; André T.; Sobrero A. F.; Mini E.; Greil R.; Di Costanzo F.; Collette L.; Cisar L.; Zhang X.; Khayat D.; Bokemeyer C.; Roth A. D.; Cunningham D. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J. Clin. Oncol. 27:3117–3125; 2009. - PubMed
-
- André T.; Boni C.; Mounedji-Boudiaf L.; Navarro M.; Tabernero J.; Hickish T.; Topham C.; Zaninelli M.; Clingan P.; Bridgewater J.; Tabah-Fisch I.; de Gramont A.; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 350:2343–2351; 2004. - PubMed
-
- André T.; Boni C.; Navarro M.; Tabernero J.; Hickish T.; Topham C.; Bonetti A.; Clingan P.; Bridgewater J.; Rivera F.; de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II and III colon cancer in the MOSAIC trial. J. Clin. Oncol. 27:3109–3116; 2009. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources