Midregional Proadrenomedullin Improves Risk Stratification beyond Surgical Risk Scores in Patients Undergoing Transcatheter Aortic Valve Replacement
- PMID: 26630012
- PMCID: PMC4667909
- DOI: 10.1371/journal.pone.0143761
Midregional Proadrenomedullin Improves Risk Stratification beyond Surgical Risk Scores in Patients Undergoing Transcatheter Aortic Valve Replacement
Abstract
Background: Conventional surgical risk scores lack accuracy in risk stratification of patients undergoing transcatheter aortic valve replacement (TAVR). Elevated levels of midregional proadrenomedullin (MR-proADM) levels are associated with adverse outcome not only in patients with manifest chronic disease states, but also in the general population.
Objectives: We investigated the predictive value of MR-proADM for mortality in an unselected contemporary TAVR population.
Methods: We prospectively included 153 patients suffering from severe aortic stenosis who underwent TAVR from September 2013 to August 2014. This population was compared to an external validation cohort of 205 patients with severe aortic stenosis undergoing TAVR. The primary endpoint was all cause mortality.
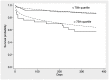
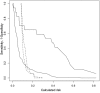
Results: During a median follow-up of 258 days, 17 out of 153 patients who underwent TAVR died (11%). Patients with MR-proADM levels above the 75th percentile (≥ 1.3 nmol/l) had higher mortality (31% vs. 4%, HR 8.9, 95% CI 3.0-26.0, P < 0.01), whereas patients with EuroSCORE II scores above the 75th percentile (> 6.8) only showed a trend towards higher mortality (18% vs. 9%, HR 2.1, 95% CI 0.8-5.6, P = 0.13). The Harrell's C-statistic was 0.58 (95% CI 0.45-0.82) for the EuroSCORE II, and consideration of baseline MR-proADM levels significantly improved discrimination (AUC = 0.84, 95% CI 0.71-0.92, P = 0.01). In bivariate analysis adjusted for EuroSCORE II, MR-proADM levels ≥1.3 nmol/l persisted as an independent predictor of mortality (HR 9.9, 95% CI (3.1-31.3), P <0.01) and improved the model's net reclassification index (0.89, 95% CI (0.28-1.59). These results were confirmed in the independent validation cohort.
Conclusions: Our study identified MR-proADM as a novel predictor of mortality in patients undergoing TAVR. In the future, MR-proADM should be added to the commonly used EuroSCORE II for better risk stratification of patients suffering from severe aortic stenosis.
Conflict of interest statement
Figures




References
-
- Arnold SV, Reynolds MR, Lei Y, Magnuson EA, Kirtane AJ, Kodali SK, et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129(25):2682–90. 10.1161/CIRCULATIONAHA.113.007477 - DOI - PMC - PubMed
-
- Duncan A, Ludman P, Banya W, Cunningham D, Marlee D, Davies S, et al. Long-term outcomes after transcatheter aortic valve replacement in high-risk patients with severe aortic stenosis: the U.K. Transcatheter Aortic Valve Implantation Registry. JACC Cardiovasc Interv. 2015;8(5):645–53. 10.1016/j.jcin.2015.01.009 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

