Understanding Programming of Fungal Iterative Polyketide Synthases: The Biochemical Basis for Regioselectivity by the Methyltransferase Domain in the Lovastatin Megasynthase
- PMID: 26630357
- PMCID: PMC4906797
- DOI: 10.1021/jacs.5b11814
Understanding Programming of Fungal Iterative Polyketide Synthases: The Biochemical Basis for Regioselectivity by the Methyltransferase Domain in the Lovastatin Megasynthase
Abstract
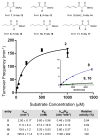
Highly reducing polyketide synthases (HR-PKSs) from fungi synthesize complex natural products using a single set of domains in a highly programmed, iterative fashion. The most enigmatic feature of HR-PKSs is how tailoring domains function selectively during different iterations of chain elongation to afford structural diversity. Using the lovastatin nonaketide synthase LovB as a model system and a variety of acyl substrates, we characterized the substrate specificity of the LovB methyltransferase (MT) domain. We showed that, while the MT domain displays methylation activity toward different β-ketoacyl groups, it is exceptionally selective toward its naturally programmed β-keto-dienyltetraketide substrate with respect to both chain length and functionalization. Accompanying characterization of the ketoreductase (KR) domain displays broader substrate specificity toward different β-ketoacyl groups. Our studies indicate that selective modifications by tailoring domains, such as the MTs, are achieved by higher kinetic efficiency on a particular substrate relative to the rate of transformation by other competing domains.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

